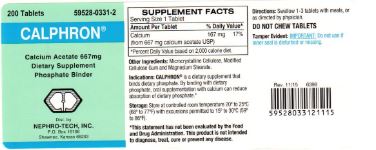
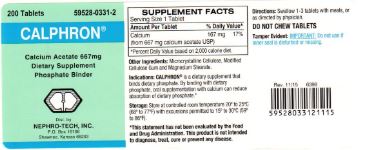
Label: CALPHRON- mineral supplement containing calcium acetate tablet
- NHRIC Code(s): 59528-0331-2
- Packager: Nephro-Tech, Inc.
- Category: DIETARY SUPPLEMENT
- DEA Schedule: None
- Marketing Status: Dietary Supplement
Drug Label Information
Updated May 12, 2016
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Principal Display Panel
- Directions
-
Dietary Supplement Phosphate Binder
Calphron is a dietary supplement that binds dietary phosphate. By binding with dietary phosphate, oral supplementation with calcium can reduce absorption of dietary phosphate.*
*This statement has not been evaluated by the Food and Drug Administration. This product is not intended to diagnose, treat, cure or prevent any disease.
-
INGREDIENTS AND APPEARANCE
CALPHRON
mineral supplement containing calcium acetate tabletProduct Information Product Type DIETARY SUPPLEMENT Item Code (Source) NHRIC:59528-0331 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CALCIUM ACETATE (UNII: Y882YXF34X) (CALCIUM CATION - UNII:2M83C4R6ZB) CALCIUM ACETATE 667 mg Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NHRIC:59528-0331-2 200 in 1 BOTTLE, PLASTIC Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date dietary supplement 04/01/2007 Supplement Facts Serving Size : Serving per Container : Amount Per Serving % Daily Value color shape size (solid drugs) 19 mm scoring 1 Labeler - Nephro-Tech, Inc. (878520485) Registrant - Nephro-Tech, Inc. (878520485) Establishment Name Address ID/FEI Business Operations Nexgen Pharma 806784679 manufacture(59528-0331)