Label: ANIPRYL- selegiline hydrochloride tablet
- NDC Code(s): 54771-8192-1, 54771-8193-1, 54771-8194-1, 54771-8195-1
- Packager: Zoetis Inc.
- Category: PRESCRIPTION ANIMAL DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Animal Drug Application
Drug Label Information
Updated December 13, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- CAUTION
-
DESCRIPTION
Anipryl (selegiline hydrochloride tablets) are white, convex tablets containing 5, 10, 15, and 30 mg of selegiline HCl. It is commonly referred to in the clinical and pharmacological literature as L-deprenyl (the levorotatory form of deprenyl HCl).
Selegiline hydrochloride is (—)-(R)-N,α-Dimethyl-N-2-propynylphenethylamine hydrochloride.
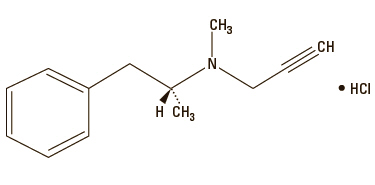
Molecular Formula: C13H17N HCl
Molecular Weight: 223.75 -
PHARMACOLOGY
Selegiline is an irreversible inhibitor of monoamine oxidase (MAO).1,2 MAOs are widely distributed throughout the body and are subclassified into 2 types, A and B, which differ in their substrate specificity and tissue distribution. Selegiline is believed to be a selective inhibitor of MAO-B at recommended dosages in the dog due to its greater affinity for type B enzyme active sites compared to type A sites.1 In CNS neurons, MAO plays a role in the catabolism of catecholamines, (dopamine, and, to a lesser extent, norepinephrine and epinephrine) and serotonin.1,2 Selegiline may have pharmacologic effects unrelated to MAO-B inhibition. There is some evidence that it may increase dopaminergic activity by other mechanisms, including increasing synthesis and release of dopamine into the synapse as well as interfering with dopamine re-uptake from the synapse.2–4 Effects resulting from selegiline administration may also be mediated through its metabolites. Two of its 3 principal metabolites, L-amphetamine and L-methamphetamine, have pharmacologic actions of their own. However, the extent to which these metabolites contribute to the effects of selegiline is unknown.
Therapeutic effects of selegiline are thought to result in part from enhanced catecholaminergic nerve function and increased dopamine levels in the CNS.5,6 The pathogenesis of the development of clinical signs associated with cognitive decline is considered to be partly a result of a decrease in the level of catecholamines in the CNS and deficiencies in neurotransmission.7 There is evidence which points to hypothalamic dopamine deficiency playing a role in the pathogenesis of pituitary dependent hyperadrenocorticism in the dog.8,9
Based upon IV administration of selegiline to 4 mixed breed female dogs, the plasma elimination half-life was estimated to be 60 ± 10 minutes (mean ± SD) and the volume of distribution at steady-state (Vss) was estimated to be 9.4 ± 1.6 L/kg (mean ± SD). The relatively large Vss suggests that the selegiline is extensively distributed to body tissues. The absolute bioavailability, F, of an oral solution was less than 10%.10
- INDICATIONS
- CONTRAINDICATIONS
-
WARNINGS
Keep out of reach of children. Not for human use.
Anipryl should not be administered at doses exceeding those recommended (0.5–2.0 mg/kg once daily).
In humans, concurrent use of MAO inhibitors with alpha-2 agonists has resulted in extreme fluctuations of blood pressure; therefore, blood pressure monitoring is recommended with concurrent use in dogs. Also, in humans, severe CNS toxicity including death has been reported with the combination of selegiline and tricyclic antidepressants, and selegiline and selective serotonin reuptake inhibitors. Although no such adverse drug interactions were reported in the clinical trials in dogs, it seems prudent to avoid the combination of Anipryl and selective serotonin reuptake inhibitors (e.g., fluoxetine) as well as Anipryl and tricyclic (e.g., clomipramine, amitriptyline, imipramine) or other antidepressants.
At least 14 days should elapse between discontinuation of Anipryl and initiation of treatment with a tricyclic antidepressant or selective serotonin reuptake inhibitor. Because of the long half-life of fluoxetine and its active
metabolites, at least 5 weeks should elapse between discontinuation of fluoxetine and initiation of treatment with Anipryl.
Concurrent use of Anipryl with ephedrine or potential MAO inhibitors, such as amitraz, is not recommended. -
PRECAUTIONS:
General
Anipryl is not recommended for other behavior problems such as aggression. In the clinical trials, 3 dogs showed an increase in aggression while on this drug. The safety and efficacy of Anipryl has not been evaluated in dogs with debilitating systemic diseases other than PDH.
The decision to prescribe Anipryl should take into consideration that the MAO system of enzymes is complex and incompletely understood and there is only a limited amount of carefully documented clinical experience with selegiline. Consequently, the full spectrum of possible responses to selegiline may not have been observed in pre-marketing evaluation of the drug. It is advisable, therefore, to observe patients carefully for atypical responses.
Endocrine function testing to confirm pituitary dependent hyperadrenocorticism should be performed prior to Anipryl administration for that condition. Anipryl is not recommended for treatment of patients with hyperadrenocorticism not of pituitary origin such as those due to an adrenal tumor or administration of glucocorticoids. If complications of PDH are evident at the time of diagnosis or emerge during Anipryl therapy, the patient should be evaluated and, if warranted, alternative therapy considered. Concurrent use of Anipryl in conjunction with other therapies of canine PDH has not been evaluated.Laboratory Tests
No specific laboratory tests are deemed essential for the management of patients on Anipryl, as response to therapy should be based on the history and physical examinations for both PDH and CDS. In clinical trials for PDH, no correlation was found between an individual patient’s clinical response and results of the low dose dexamethasone suppression (LDDS) test. There was no evidence of adrenal insufficiency in these trials.
In the 12 week clinical trial for CDS, a small number of dogs had a drop in hematocrit; some dropping within the normal range and some dropping below 37%. The clinical significance of this is unknown at this time. It is advisable to conduct a thorough physical examination and to consider appropriate laboratory tests to establish hematological and serum biochemical baseline data prior to administration of Anipryl. -
ADVERSE REACTIONS
In clinical trials, 404 dogs treated with Anipryl for as long as 18 months were monitored for the occurrence of adverse events. Many of the observations listed in the following table may be associated with the underlying disease (PDH or CDS), the advanced age of the patients or the development of unrelated concurrent disease. One index of relative importance, however, is whether or not a reaction caused treatment discontinuation. Eighteen dogs (4%) experienced one or more of the following adverse events that led either to discontinuation of therapy with Anipryl, dismissal from the study, or a reduction in dose: restlessness/agitation, vomiting, disorientation, diarrhea, diminished hearing, possible drug interaction (weakness, confusion, incoordination and "seizure-like" activity while being treated concurrently with metronidazole, prednisone, and trimethoprim sulfa), increase in destructive behavior in a dog with separation anxiety, anorexia, anemia, stiffness and polydipsia.
Percentage of Dogs with Adverse Events Reported in Clinical Field Trials Adverse Event Anipryl (n=404) Placebo (n=67) vomiting 26% 21% diarrhea 18% 10% hyperactive/restless* 12% 6% anorexia 8% 1% neurologic** 6% 1% lethargy 6% 1% salivation 5% 4% urinary tract infection 4% 1% pruritus/dermatologic 4% 1% weakness 4% 0 pale gums 3% 1% polyuria/polydipsia 3% 1% weight loss 3% 0 diminished hearing 2% 0 panting 2% 1% cardiovascular/respiratory*** 2% 0 licking 2% 1% *This includes hyperactivity, irritability, abnormal repetitive movements, anxiousness, and restlessness.
**This includes ataxia, incoordination, staggering, disorientation, decreased proprioception, and seizure.
***This includes heart murmurs, tachycardia, collapse, dyspnea, pleural effusion, and sneezing. -
DOSAGE AND ADMINISTRATION:
CDS
The recommended dosage for oral administration for the control of clinical signs associated with CDS is 0.5–1.0 mg/kg once daily, preferably administered in the morning. Initially, dogs should be dosed to the nearest whole tablet. Adjustments should then be made based on response and tolerance to the drug.
PDH
The recommended dosage for the control of clinical signs associated with canine PDH is 1.0 mg/kg once daily, preferably administered in the morning. If no improvement is observed after 2 months of therapy, dosage may be increased to a maximum of 2.0 mg/kg once daily. If no improvement is seen after 1 month at the higher dose or if at any time clinical signs progress, the dog should be re-evaluated. In dogs whose clinical signs of PDH progress despite Anipryl therapy in the absence of concurrent disease, alternate therapy should be considered.
Dogs should be monitored closely for possible adverse events associated with any increase in dose.Clinical Use of Anipryl in CDS
CDS is an age-related deterioration of cognitive abilities characterized by behavioral changes not wholly attributable to a general medical condition such as neoplasia, infection, or organ failure. CDS is typified by multiple cognitive impairments which affect the dog's function. In clinical trials, the observed behavioral changes associated with CDS in older dogs included: disorientation, decreased activity level, abnormal sleep/wake cycles, loss of housetraining, decreased or altered responsiveness to family members, and decreased or altered greeting behavior. In clinical trials, Anipryl was shown to be effective in controlling clinical signs associated with CDS. After 4 weeks of treatment, dogs treated with Anipryl showed significant improvement when compared to placebo-treated controls in sleeping patterns, house-training, and activity level. Some dogs showed increased improvement up to 3 months, however, onset, duration and magnitude of response varied with individual dogs.
The diagnosis of CDS in dogs is a diagnosis of exclusion, based on thorough behavioral and medical histories, in conjunction with appropriate diagnostic work-up and testing.11 Periodic patient monitoring to evaluate the response and tolerance to the drug and for the presence of concurrent or new disease is recommended.
Clinical Use of Anipryl in PDH
Clinical signs of PDH seen in clinical trials included panting, reduced activity, polydipsia, polyuria, changes in sleep patterns, altered appetite, obesity, alopecia, abdominal distention, reduced skin elasticity, thin skin, poor hair growth, pyoderma, decreased responsiveness to attention, and decreased enthusiasm of greeting. In clinical studies involving 125 evaluable cases of naturally occurring PDH, Anipryl was shown to be effective in controlling clinical signs associated with the disease. On physical examination, abdominal distention was the parameter which most consistently improved following treatment with Anipryl. Based on owner assessments, activity level was the parameter most consistently evaluated as "improved." Approximately 60% of the dogs were evaluated by the veterinarians and owners to be "slightly improved" to "improved" after 1 month of Anipryl therapy. By month 2, veterinarians reported that approximately 77% were "slightly improved" to "improved." Approximately 20% of dogs did not respond to Anipryl and were deemed treatment failures.
Those dogs that responded to Anipryl tended to do so within 1–2 months after treatment was initiated. Response to therapy varied between patients with some dogs showing improvement in all presenting clinical signs and others showing improvement in only 1–2 parameters. Duration of response was also variable, with some dogs continuing on Anipryl for over 1 year with good control of clinical signs and others showing an initial response to therapy only to be followed within several months by recurrence of clinical signs of PDH. There was no correlation demonstrated between an individual dog's clinical response to Anipryl and that dog's low dose dexamethasone suppression test results, therefore, monitoring should be based on history and physical examination findings.
SAFETY
In a laboratory safety study, Anipryl was administered orally to healthy adult beagles once daily for 6 months at doses of 0, 1, 2, 3, or 6 mg/kg (0.5x, 1x, 1.5x and 3x the maximum recommended daily dose of 2.0 mg/kg). The drug was demonstrated to be safe at the recommended dose range of 0.5–2.0 mg/kg. The following statistically significant clinical observations were noted in dogs in the 1.5x and 3x group: salivation, decreased pupillary response, and decreased body weight despite normal to increased feed consumption. Additional reactions seen at the 3x dose included panting, decreased skin elasticity (dehydration) and stereotypic behaviors, i.e., weaving (repetitive left to right movement) in the cage. This repetitive movement started several hours after dosing but was no longer present at the time of the next morning dose. There were no changes noted in blood pressure, heart rate and ECG parameters, nor were there any ophthalmic changes.
-
REFERENCES
1. Milgram NW, Ivy GO, Murphy MP, et al: Effects of chronic oral administration of L-deprenyl in the dog. Pharmacol Biochem Behav 51:421–428, 1995.
2. Heinonen EH, Lammintausta R: A review of the pharmacology of selegiline. Acta Neurol Scand 84:(suppl. 136): 44–59, 1991.
3. Knoll J: R-(-)Deprenyl (selegiline, Movergan®) facilitates the activity of the nigrostriatal dopaminergic neuron. J Neural Transm (suppl. 25):45–66, 1987.
4. Heikkila RE, Cabbat FS, Manzino L, et al: Potentiation by deprenil of l-dopa induced circling in nigral-lesioned rats. Pharmacol Biochem Behav 15:75–79, 1981.
5. Knoll J, Miklya I, Knoll B, et al: (-)Deprenyl and (-)1-phenyl-2-propylaminopentane, [(-) PPAP], act primarily as potent stimulants of action potential - transmitter release coupling in the catecholaminergic neurons. Life Sci 58:817–827, 1996.
6. Riederer P, Youdim MBH, Rausch WD, et al: On the mode of action of L-deprenyl in the human central nervous system. J Neural Transm 43:217–226, 1978.
7. Arnsten, AFT: Catecholamine mechanisms in age-related cognitive decline. Neurobiol Aging 14:639–641, 1993.
8. Peterson ME, Palkovits M, Chiueh CC, et al: Biogenic amine and corticotropin-releasing factor concentrations in hypothalamic paraventricular nucleus and biogenic amine levels in the median eminence of normal dogs, chronic dexamethasone-treated dogs, and dogs with naturally-occurring pituitary-dependent hyperadrenocorticism (Canine Cushing's Disease). J Neuroendocrinology 1:169–171, 1989.
9. Bruyette DS, Ruehl WW, Entriken TL, et al: Treating canine pituitary-dependent hyperadrenocorticism with L-deprenyl. Veterinary Medicine 92:711–727, 1997.
10. Mahmood I, Peters DK, Mason WD: The pharmacokinetics and absolute bioavailability of selegiline in the dog. Biopharm Drug Dispos 15:653–664, 1994.
11. Ruehl WW, Hart BL: Canine cognitive dysfunction, in Dodman N, Shuster L (eds): Psychopharmacology of Animal Behavior Disorders. Cambridge, MA, Blackwell Science, Inc. 1998.
- HOW SUPPLIED
- STORAGE
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 5 mg Tablet Blister Pack Box
- PRINCIPAL DISPLAY PANEL - 10 mg Tablet Blister Pack Box
- PRINCIPAL DISPLAY PANEL - 15 mg Tablet Blister Pack Box
- PRINCIPAL DISPLAY PANEL - 30 mg Tablet Blister Pack Box
-
INGREDIENTS AND APPEARANCE
ANIPRYL
selegiline hydrochloride tabletProduct Information Product Type PRESCRIPTION ANIMAL DRUG Item Code (Source) NDC:54771-8192 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SELEGILINE HYDROCHLORIDE (UNII: 6W731X367Q) (SELEGILINE - UNII:2K1V7GP655) SELEGILINE HYDROCHLORIDE 5 mg Product Characteristics Color white Score 2 pieces Shape OVAL Size 8mm Flavor Imprint Code 5;MG;on;one;side;ANIPRYL;on;the;other Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:54771-8192-1 1 in 1 BOX 1 30 in 1 BLISTER PACK Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NADA NADA141080 05/10/1995 ANIPRYL
selegiline hydrochloride tabletProduct Information Product Type PRESCRIPTION ANIMAL DRUG Item Code (Source) NDC:54771-8193 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SELEGILINE HYDROCHLORIDE (UNII: 6W731X367Q) (SELEGILINE - UNII:2K1V7GP655) SELEGILINE HYDROCHLORIDE 10 mg Product Characteristics Color white Score 2 pieces Shape PENTAGON (5 sided) Size 9mm Flavor Imprint Code 10;MG;ANIPRYL Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:54771-8193-1 1 in 1 BOX 1 30 in 1 BLISTER PACK Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NADA NADA141080 05/10/1995 ANIPRYL
selegiline hydrochloride tabletProduct Information Product Type PRESCRIPTION ANIMAL DRUG Item Code (Source) NDC:54771-8194 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SELEGILINE HYDROCHLORIDE (UNII: 6W731X367Q) (SELEGILINE - UNII:2K1V7GP655) SELEGILINE HYDROCHLORIDE 15 mg Product Characteristics Color white Score 2 pieces Shape OCTAGON (8 sided) Size 8mm Flavor Imprint Code 15;MG;ANIPRYL Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:54771-8194-1 1 in 1 BOX 1 30 in 1 BLISTER PACK Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NADA NADA141080 05/10/1995 ANIPRYL
selegiline hydrochloride tabletProduct Information Product Type PRESCRIPTION ANIMAL DRUG Item Code (Source) NDC:54771-8195 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SELEGILINE HYDROCHLORIDE (UNII: 6W731X367Q) (SELEGILINE - UNII:2K1V7GP655) SELEGILINE HYDROCHLORIDE 30 mg Product Characteristics Color white Score 2 pieces Shape HEXAGON (6 sided) Size 10mm Flavor Imprint Code 30;MG;ANIPRYL Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:54771-8195-1 1 in 1 BOX 1 30 in 1 BLISTER PACK Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NADA NADA141080 05/10/1995 Labeler - Zoetis Inc. (828851555)









