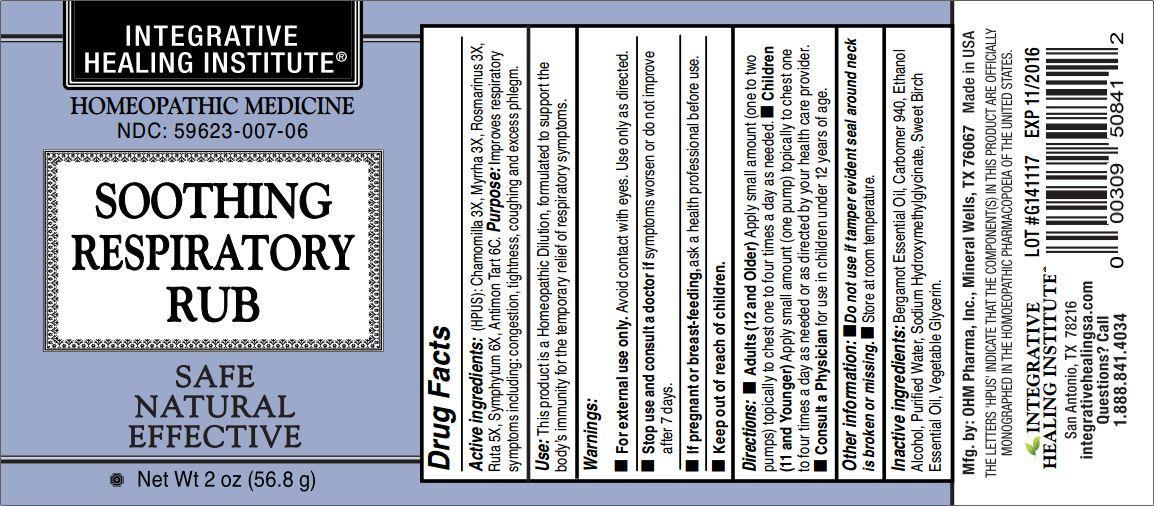
Label: SOOTHING RESPIRATORY RUB- chamomilla, myrrha, rosmarinus, ruta, symphytum, antimon tart. gel
- NDC Code(s): 59623-007-06
- Packager: Integrative Healing Institute, LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved homeopathic
DISCLAIMER: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Drug Label Information
Updated December 29, 2021
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- PURPOSE
- INDICATIONS & USAGE
- WARNINGS
-
DOSAGE & ADMINISTRATION
Directions:
- Adults (12 and Older) Apply small amount (one to two pumps) topically to chest one to four times a day as needed.
- Children (11 and Younger) Apply small amount (one pump) topically to chest one to four times a day as needed or as directed by your health care provider.
- Consult a Physician for use in children under 12 years of age.
- STORAGE AND HANDLING
- INACTIVE INGREDIENT
- OTHER SAFETY INFORMATION
- QUESTIONS
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
SOOTHING RESPIRATORY RUB
chamomilla, myrrha, rosmarinus, ruta, symphytum, antimon tart. gelProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:59623-007 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MATRICARIA RECUTITA (UNII: G0R4UBI2ZZ) (MATRICARIA RECUTITA - UNII:G0R4UBI2ZZ) MATRICARIA RECUTITA 3 [hp_X] in 1 g MYRRH (UNII: JC71GJ1F3L) (MYRRH - UNII:JC71GJ1F3L) MYRRH 3 [hp_X] in 1 g ROSMARINUS OFFICINALIS FLOWERING TOP (UNII: 8JM482TI79) (ROSMARINUS OFFICINALIS FLOWERING TOP - UNII:8JM482TI79) ROSMARINUS OFFICINALIS FLOWERING TOP 3 [hp_X] in 1 g RUTA GRAVEOLENS FLOWERING TOP (UNII: N94C2U587S) (RUTA GRAVEOLENS FLOWERING TOP - UNII:N94C2U587S) RUTA GRAVEOLENS FLOWERING TOP 5 [hp_X] in 1 g COMFREY ROOT (UNII: M9VVZ08EKQ) (COMFREY ROOT - UNII:M9VVZ08EKQ) COMFREY ROOT 6 [hp_X] in 1 g ANTIMONY POTASSIUM TARTRATE (UNII: DL6OZ476V3) (ANTIMONY CATION (3+) - UNII:069647RPT5) ANTIMONY POTASSIUM TARTRATE 6 [hp_C] in 1 g Inactive Ingredients Ingredient Name Strength BERGAMOT OIL (UNII: 39W1PKE3JI) CARBOMER HOMOPOLYMER TYPE C (ALLYL PENTAERYTHRITOL CROSSLINKED) (UNII: 4Q93RCW27E) ALCOHOL (UNII: 3K9958V90M) WATER (UNII: 059QF0KO0R) SODIUM HYDROXYMETHYLGLYCINATE (UNII: DIG6BWZ9XT) METHYL SALICYLATE (UNII: LAV5U5022Y) GLYCERIN (UNII: PDC6A3C0OX) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:59623-007-06 56.8 g in 1 BOTTLE, PUMP; Type 0: Not a Combination Product 02/18/2015 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 02/18/2015 Labeler - Integrative Healing Institute, LLC (938638595)