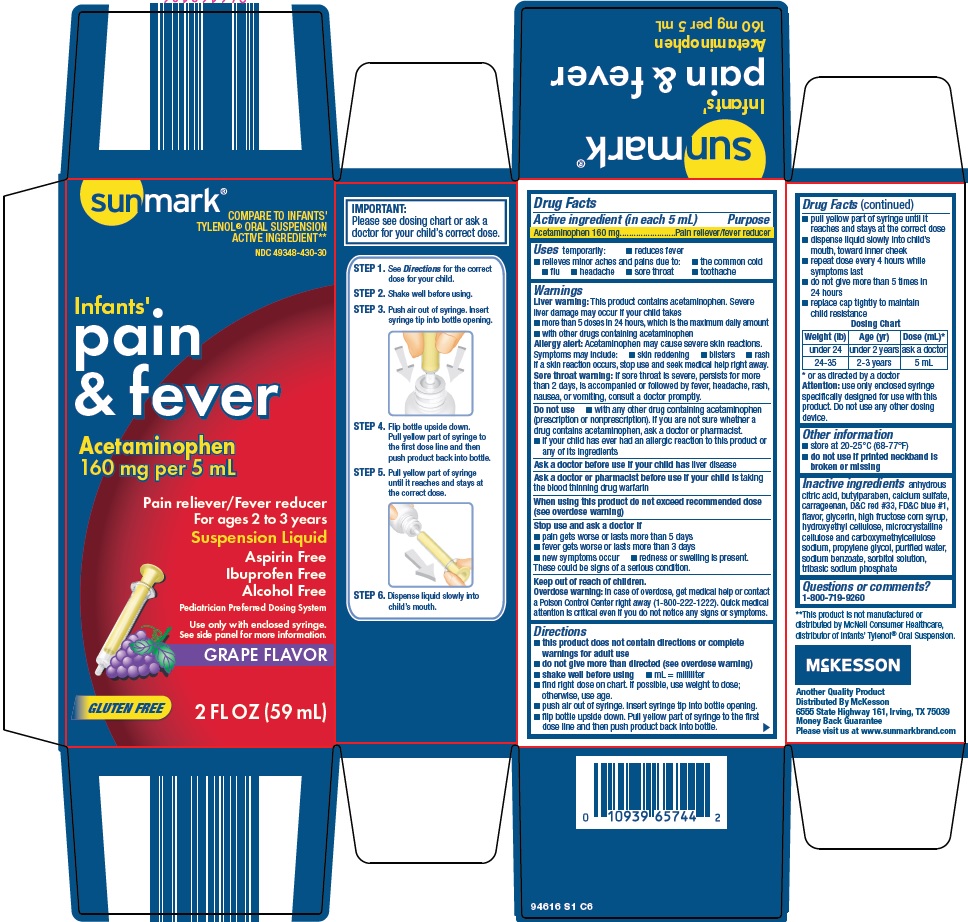
Label: SUNMARK INFANTS PAIN AND FEVER- acetaminophen suspension
- NDC Code(s): 49348-430-30
- Packager: Strategic Sourcing Services LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated January 2, 2020
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredient (in each 5 mL)
- Purpose
- Uses
-
Warnings
Liver warning: This product contains acetaminophen. Severe liver damage may occur if your child takes
- •
- more than 5 doses in 24 hours, which is the maximum daily amount
- •
- with other drugs containing acetaminophen
Allergy alert: Acetaminophen may cause severe skin reactions. Symptoms may include:
- •
- skin reddening
- •
- blisters
- •
- rash
If a skin reaction occurs, stop use and seek medical help right away.
Sore throat warning: If sore throat is severe, persists for more than 2 days, is accompanied or followed by fever, headache, rash, nausea, or vomiting, consult a doctor promptly.
Do not use
- •
- with any other drug containing acetaminophen (prescription or nonprescription). If you are not sure whether a drug contains acetaminophen, ask a doctor or pharmacist.
- •
- if your child has ever had an allergic reaction to this product or any of its ingredients
-
Directions
- •
- this product does not contain directions or complete warnings for adult use
- •
- do not give more than directed (see overdose warning)
- •
- shake well before using
- •
- mL = milliliter
- •
- find right dose on chart. If possible, use weight to dose; otherwise, use age.
- •
- push air out of syringe. Insert syringe tip into bottle opening.
- •
- flip bottle upside down. Pull yellow part of syringe to the first dose line and then push product back into bottle.
- •
- pull yellow part of syringe until it reaches and stays at the correct dose
- •
- dispense liquid slowly into child’s mouth, toward inner cheek
- •
- repeat dose every 4 hours while symptoms last
- •
- do not give more than 5 times in 24 hours
- •
- replace cap tightly to maintain child resistance
Dosing Chart
Weight (lb)
Age (yr)
Dose (mL)*
under 24
under 2 years
ask a doctor
24-35
2-3 years
5 mL
* or as directed by a doctor
Attention: use only enclosed syringe specifically designed for use with this product. Do not use any other dosing device.
-
Inactive ingredients
anhydrous citric acid, butylparaben, calcium sulfate, carrageenan, D&C red #33, FD&C blue #1, flavor, glycerin, high fructose corn syrup, hydroxyethyl cellulose, microcrystalline cellulose and carboxymethylcellulose sodium, propylene glycol, purified water, sodium benzoate, sorbitol solution, tribasic sodium phosphate
- Questions or comments?
-
Package/Label Principal Display Panel
COMPARE TO INFANTS’ TYLENOL® ORAL SUSPENSION ACTIVE INGREDIENT
Infants’
pain & fever
Acetaminophen 160 mg per 5 mL
Pain reliever / Fever reducer
For ages 2 to 3 years
Suspension Liquid
Aspirin Free
Ibuprofen Free
Alcohol Free
Pediatrician Preferred Dosing System
Use only with enclosed syringe.
See side panel for more information.
GRAPE FLAVOR
GLUTEN FREE
2 FL OZ (59 mL)
-
INGREDIENTS AND APPEARANCE
SUNMARK INFANTS PAIN AND FEVER
acetaminophen suspensionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:49348-430 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ACETAMINOPHEN (UNII: 362O9ITL9D) (ACETAMINOPHEN - UNII:362O9ITL9D) ACETAMINOPHEN 160 mg in 5 mL Inactive Ingredients Ingredient Name Strength ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) BUTYLPARABEN (UNII: 3QPI1U3FV8) CALCIUM SULFATE, UNSPECIFIED FORM (UNII: WAT0DDB505) CARRAGEENAN (UNII: 5C69YCD2YJ) D&C RED NO. 33 (UNII: 9DBA0SBB0L) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) GLYCERIN (UNII: PDC6A3C0OX) HIGH FRUCTOSE CORN SYRUP (UNII: XY6UN3QB6S) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) CARBOXYMETHYLCELLULOSE SODIUM, UNSPECIFIED FORM (UNII: K679OBS311) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) WATER (UNII: 059QF0KO0R) SODIUM BENZOATE (UNII: OJ245FE5EU) SORBITOL (UNII: 506T60A25R) SODIUM PHOSPHATE, TRIBASIC (UNII: A752Q30A6X) Product Characteristics Color PURPLE Score Shape Size Flavor GRAPE Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:49348-430-30 1 in 1 CARTON 09/26/2011 1 59 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part343 09/26/2011 Labeler - Strategic Sourcing Services LLC (116956644)