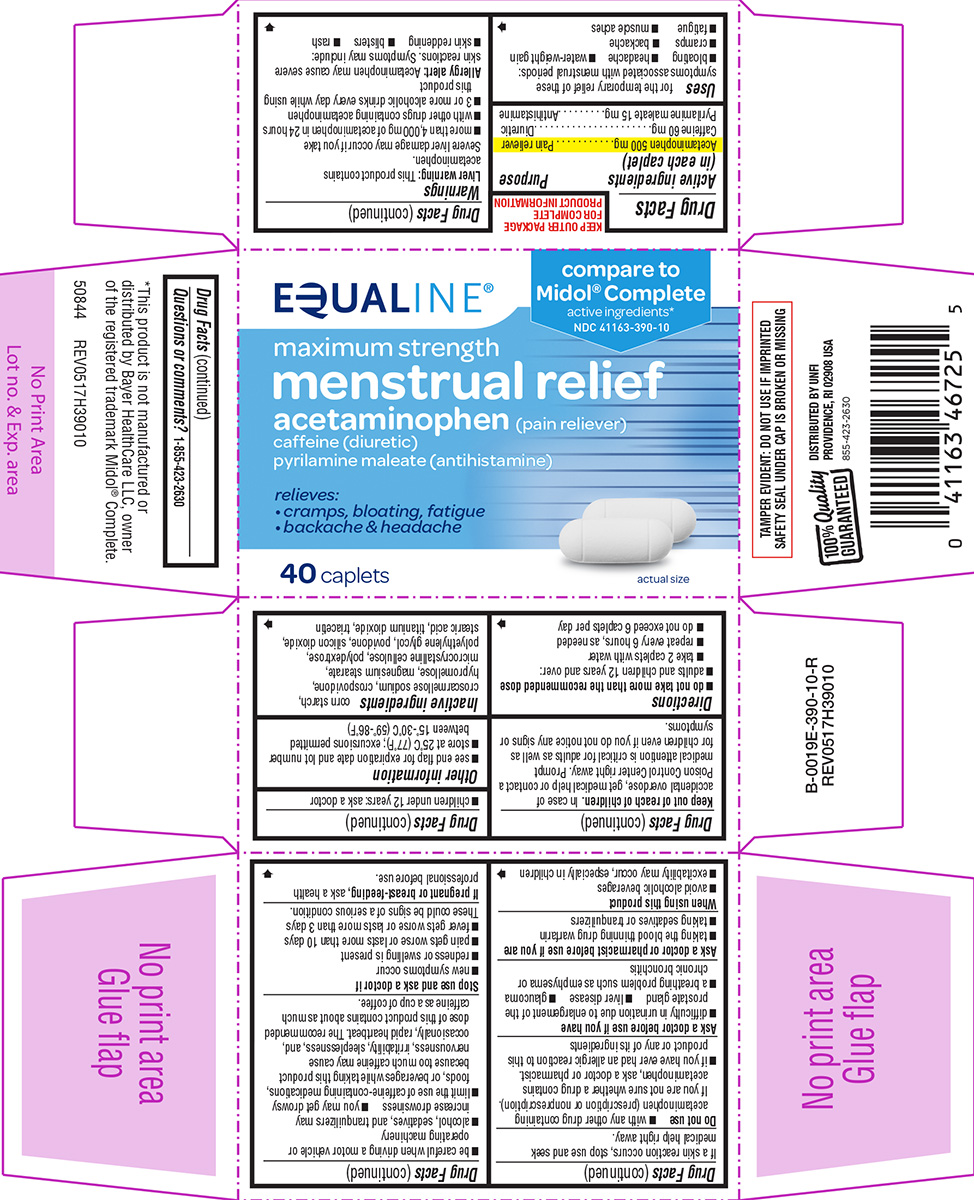
Label: MENSTRUAL RELIEF MAXIMUM STRENGTH- acetaminophen, caffeine and pyrilamine maleate tablet, film coated
- NDC Code(s): 41163-390-10
- Packager: United Natural Foods, Inc. dba UNFI
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated January 22, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredients (in each caplet)
- Purpose
- Uses
-
Warnings
Liver warning: This product contains acetaminophen. Severe liver damage may occur if you take
- more than 4,000 mg of acetaminophen in 24 hours
- with other drugs containing acetaminophen
- 3 or more alcoholic drinks every day while using this product
Allergy alert: Acetaminophen may cause severe skin reactions. Symptoms may include:
- skin reddening
- blisters
- rash
If a skin reaction occurs, stop use and seek medical help right away.
Do not use
- with any other drug containing acetaminophen (prescription or nonprescription). If you are not sure whether a drug contains acetaminophen, ask a doctor or pharmacist.
- if you have ever had an allergic reaction to this product or any of its ingredients
Ask a doctor before use if you have
- difficulty in urination due to enlargement of the prostate gland
- liver disease
- glaucoma
- a breathing problem such as emphysema or chronic bronchitis
Ask a doctor or pharmacist before use if you are
- taking the blood thinning drug warfarin
- taking sedatives or tranquilizers
When using this product
- avoid alcoholic beverages
- excitability may occur, especially in children
- be careful when driving a motor vehicle or operating machinery
- alcohol, sedatives, and tranquilizers may increase drowsiness
- you may get drowsy
- limit the use of caffeine-containing medications, foods, or beverages while taking this product because too much caffeine may cause nervousness, irritability, sleeplessness, and, occasionally, rapid heartbeat. The recommended dose of this product contains about as much caffeine as a cup of coffee.
- Directions
- Other information
- Inactive ingredients
- Questions or comments?
-
Principal Display Panel
EQUALINE®
compare to
Midol® Complete
active ingredients*NDC 41163-390-10
maximum strength
menstrual reliefacetaminophen (pain reliever)
caffeine (diuretic)
pyrilamine maleate (antihistamine)relieves:
• cramps, bloating, fatigue
• backache & headache40 caplets
actual size
TAMPER EVIDENT: DO NOT USE IF IMPRINTED
SAFETY SEAL UNDER CAP IS BROKEN OR MISSING
*This product is not manufactured or
distributed by Bayer HealthCare LLC, owner
of the registered trademark Midol® Complete.
50844 REV0517H39010100% Quality GUARANTEED
DISTRIBUTED BY UNFI
PROVIDENCE, RI 02908 USA855-423-2630
Equaline 44-390
-
INGREDIENTS AND APPEARANCE
MENSTRUAL RELIEF MAXIMUM STRENGTH
acetaminophen, caffeine and pyrilamine maleate tablet, film coatedProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:41163-390 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ACETAMINOPHEN (UNII: 362O9ITL9D) (ACETAMINOPHEN - UNII:362O9ITL9D) ACETAMINOPHEN 500 mg CAFFEINE (UNII: 3G6A5W338E) (CAFFEINE - UNII:3G6A5W338E) CAFFEINE 60 mg PYRILAMINE MALEATE (UNII: R35D29L3ZA) (PYRILAMINE - UNII:HPE317O9TL) PYRILAMINE MALEATE 15 mg Inactive Ingredients Ingredient Name Strength STARCH, CORN (UNII: O8232NY3SJ) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) CROSPOVIDONE, UNSPECIFIED (UNII: 2S7830E561) HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) MAGNESIUM STEARATE (UNII: 70097M6I30) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) POLYDEXTROSE (UNII: VH2XOU12IE) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) POVIDONE, UNSPECIFIED (UNII: FZ989GH94E) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) STEARIC ACID (UNII: 4ELV7Z65AP) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) TRIACETIN (UNII: XHX3C3X673) Product Characteristics Color white Score no score Shape OVAL Size 17mm Flavor Imprint Code 44;390 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:41163-390-10 1 in 1 CARTON 04/29/2002 1 40 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M013 04/29/2002 Labeler - United Natural Foods, Inc. dba UNFI (943556183) Establishment Name Address ID/FEI Business Operations LNK International, Inc. 038154464 pack(41163-390) Establishment Name Address ID/FEI Business Operations LNK International, Inc. 832867837 manufacture(41163-390) , pack(41163-390) Establishment Name Address ID/FEI Business Operations LNK International, Inc. 832867894 manufacture(41163-390) Establishment Name Address ID/FEI Business Operations LNK International, Inc. 967626305 pack(41163-390) Establishment Name Address ID/FEI Business Operations LNK International, Inc. 117025878 manufacture(41163-390)