Label: ANTI-AGING COMPLEX SPF 30 MERLE NORMAN- avobenzone, homosalate, octisalate, octocrylene, oxybenzone emulsion
- NDC Code(s): 57627-149-01, 57627-149-02
- Packager: Merle Norman Cosmetics, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated January 27, 2020
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
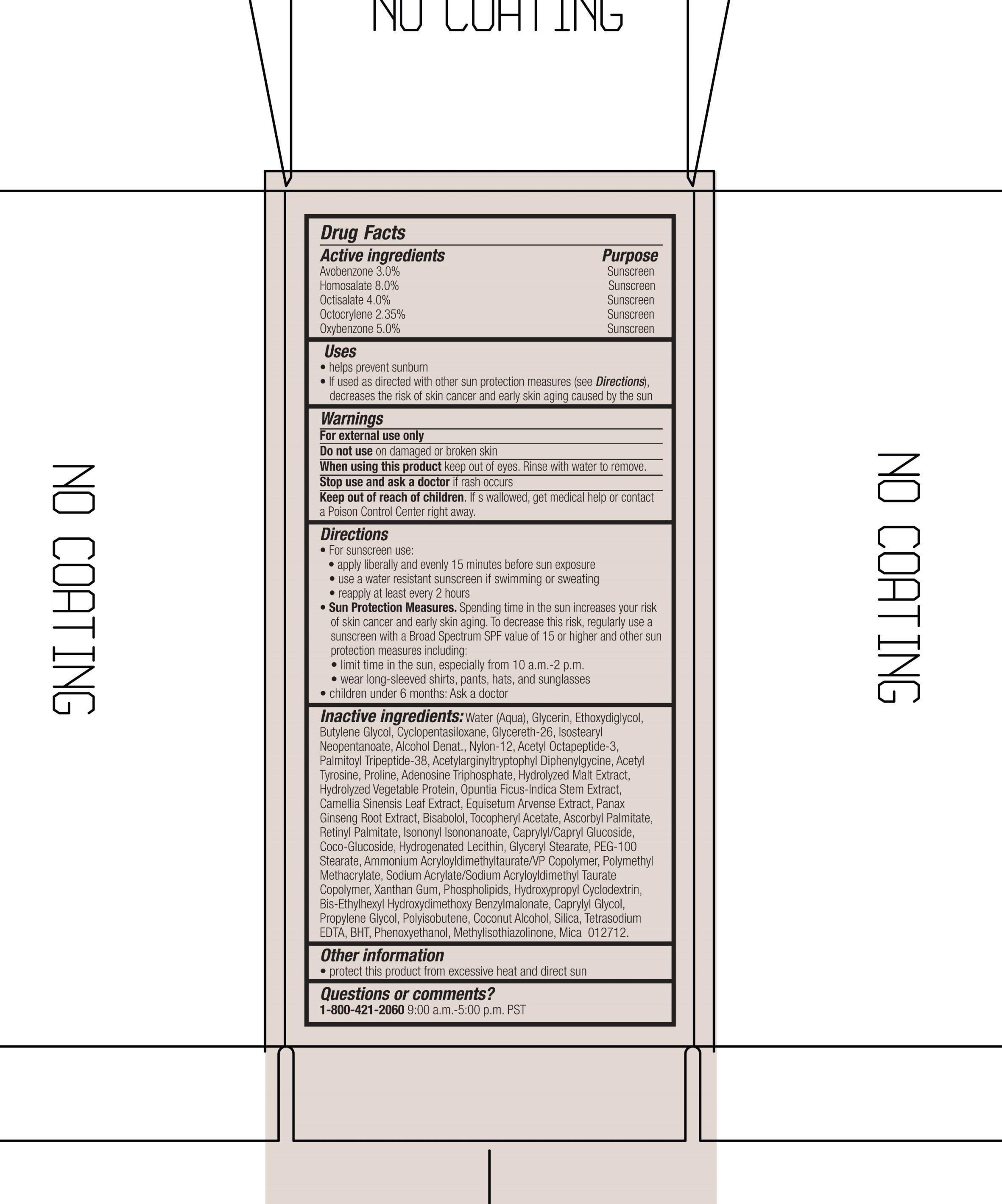
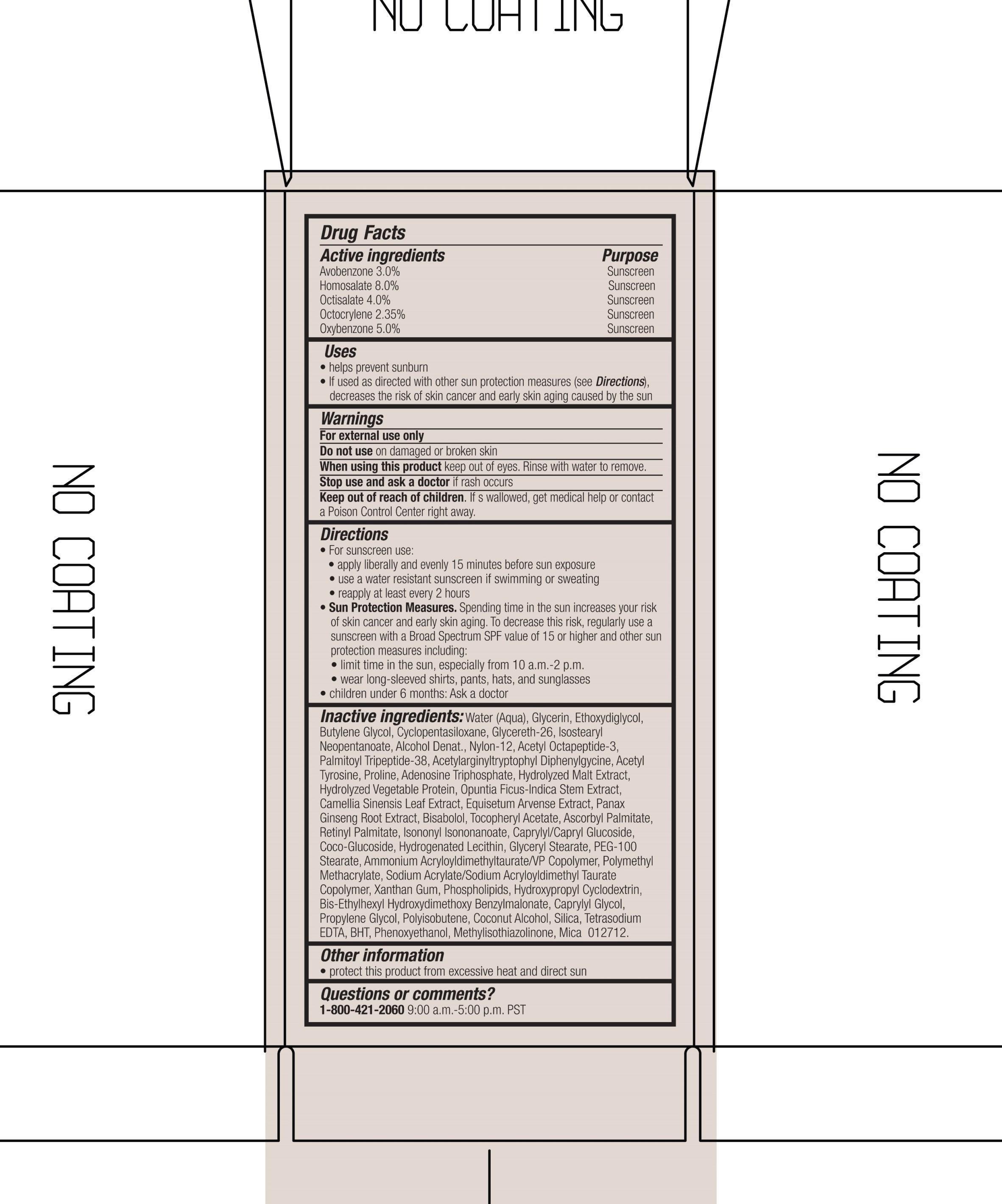
ACTIVE INGREDIENT
Active Ingredients purpose
Avobenzone 3.0% Sunscreen
Homosalate 8.0% Sunscreen
Octisalate 4.0 % Sunscreen
Octocrylene 2.35% Sunscreen
Oxybenzone 5.0% Sunscreen
Uses
Helps prevent sunburn
If used as directed with other sun protection measures (see directions), decreases the risk of skin cancer and early skin aging caused by the sun.
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
Stop use and ask a doctor if rash occurs.
Warnings
For external use only
Do not use on damaged or broken skin
When using this product, keep out of eyes. Rinse with water to remove.
Directions
for Sunscreen use
-Apply Liberally and evenly 15 minutes before sun exposure
-Use a water resistant sunscreen if swimming or sweating
-Reapply at least every 2 hours
Sun Protection Measures. Spending time in the sun increases your risk of skn cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum value of 15 or higher and other sun protection measures including:
-limit time in the sun, especially from 10 a.m. to 2 p.m.
- wear long-sleeved shirts, pants, hats and and sunglasses
Children under 6 months: Ask a doctor
Inactive ingredients: water(aqua), glycerin, ethoxydiglycol, butylene glycol, cyclopentasiloxane, glycereth-26, isostearyl neopentanoate, alcohol denat., nylon-12, acetyl octapeptide-3, palmitoyl tripeptide-38, acetylarginyltryptophyl diphenylglycine, acetyl tyrosine, proline, adenosine triphosphate, hydrolyzed malt extract, hydrolyzed vegetable protein, optunia ficus-indica stem extract, camellia sinensis leaf extract, equisetum arvense extract, panax ginseng root extract, bisabolol, tocopheryl acetate, ascorbyl palmitate, retinyl palmitate, isononyl isononanoate, caprylyl/capryl glucoside, coco-glucoside, hydrogenated lecithin, glyceryl stearate, peg-100 stearate, ammonium acryloyldimethyltaurate/VP copolymer, polymethyl methacrylate, sodium acrylate/sodium acryloyldimethyl taurate copolymer, xanthan gum, phospholipids, hydroxypropyl cyclodextrin, bis-ethylhexyl hydroxydimethoxy benzylmalonate, caprylyl glycol, propylene glycol, polyisobutene, coconut alcohol, silica, tetrasodium EDTA, BHT, phenoxyethanol, methylisothiazolinone, mica
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
ANTI-AGING COMPLEX SPF 30 MERLE NORMAN
avobenzone, homosalate, octisalate, octocrylene, oxybenzone emulsionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:57627-149 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength AVOBENZONE (UNII: G63QQF2NOX) (AVOBENZONE - UNII:G63QQF2NOX) AVOBENZONE 0.93 g in 31 g HOMOSALATE (UNII: V06SV4M95S) (HOMOSALATE - UNII:V06SV4M95S) HOMOSALATE 2.48 g in 31 g OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 1.24 g in 31 g OCTOCRYLENE (UNII: 5A68WGF6WM) (OCTOCRYLENE - UNII:5A68WGF6WM) OCTOCRYLENE 0.729 g in 31 g OXYBENZONE (UNII: 95OOS7VE0Y) (OXYBENZONE - UNII:95OOS7VE0Y) OXYBENZONE 1.55 g in 31 g Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) GLYCERIN (UNII: PDC6A3C0OX) DIETHYLENE GLYCOL MONOETHYL ETHER (UNII: A1A1I8X02B) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) GLYCERETH-26 (UNII: NNE56F2N14) ISOSTEARYL NEOPENTANOATE (UNII: 411THY156Q) NYLON-12 (UNII: 446U8J075B) ACETYL OCTAPEPTIDE-3 (UNII: 8K14HJF88S) N-ACETYLTYROSINE (UNII: DA8G610ZO5) PROLINE (UNII: 9DLQ4CIU6V) ADENOSINE TRIPHOSPHATE (UNII: 8L70Q75FXE) BARLEY MALT SYRUP (UNII: 22P8DKP670) OPUNTIA FICUS-INDICA STEM (UNII: MUD8892KHL) GREEN TEA LEAF (UNII: W2ZU1RY8B0) EQUISETUM ARVENSE TOP (UNII: 1DP6Y6B65Z) ASIAN GINSENG (UNII: CUQ3A77YXI) LEVOMENTHOL (UNII: BZ1R15MTK7) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) ASCORBYL PALMITATE (UNII: QN83US2B0N) VITAMIN A PALMITATE (UNII: 1D1K0N0VVC) ISONONYL ISONONANOATE (UNII: S4V5BS6GCX) CAPRYLYL/CAPRYL OLIGOGLUCOSIDE (UNII: E00JL9G9K0) COCO GLUCOSIDE (UNII: ICS790225B) HYDROGENATED SOYBEAN LECITHIN (UNII: H1109Z9J4N) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) PEG-100 STEARATE (UNII: YD01N1999R) AMMONIUM ACRYLOYLDIMETHYLTAURATE/VP COPOLYMER (UNII: W59H9296ZG) SODIUM ACRYLATE/SODIUM ACRYLOYLDIMETHYLTAURATE COPOLYMER (4000000 MW) (UNII: 1DXE3F3OZX) XANTHAN GUM (UNII: TTV12P4NEE) EGG PHOSPHOLIPIDS (UNII: 1Z74184RGV) BIS-ETHYLHEXYL HYDROXYDIMETHOXY BENZYLMALONATE (UNII: 7D4Q5YJ8NV) CAPRYLYL GLYCOL (UNII: 00YIU5438U) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) POLYISOBUTYLENE (1000 MW) (UNII: 5XB3A63Y52) COCONUT ALCOHOL (UNII: 13F4MW8Y9K) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) EDETATE SODIUM (UNII: MP1J8420LU) BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) PHENOXYETHANOL (UNII: HIE492ZZ3T) METHYLISOTHIAZOLINONE (UNII: 229D0E1QFA) MICA (UNII: V8A1AW0880) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:57627-149-02 1 in 1 CARTON 11/08/2012 1 NDC:57627-149-01 31 g in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 11/08/2012 Labeler - Merle Norman Cosmetics, Inc. (008479388) Registrant - Merle Norman Cosmetics, Inc. (008479388) Establishment Name Address ID/FEI Business Operations Merle Norman Cosmetics, Inc. 008479388 manufacture(57627-149)