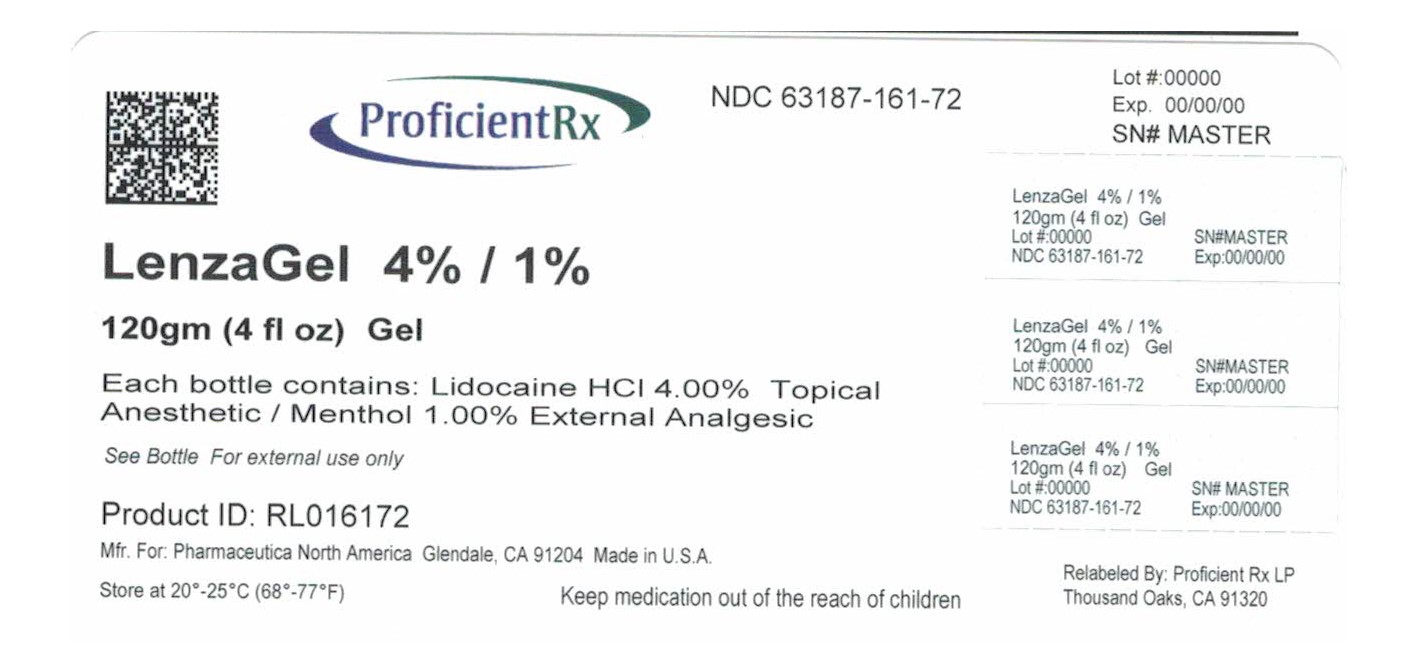
LENZAGEL- lidocaine hydrochloride, menthol gel
Proficient Rx LP
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
LenzaGel
Warnings
- •
- For external use only
- •
- Avoid contact with eyes
- •
- Do not apply to open wounds or damaged skin.
- •
- If symptoms persist for more than seven days, discontinue use and consult physician.
Other Ingredients:
Aloe Barbadensis Leaf (Aloe Vera Juice) Gel, Aqua (Deionized Water), Arnica Montana Extract, Boswellia Serrata Extract, Camellia Sinensis Leaf (Green Tea) Extract, Carbomer, Ethylhexylglycerin, Glycerin, Isopropyl Myristate, PEG-8, Phenoxyethanol, Polysorbate-80, Sodium Lauryl Sulfate, Triethanolamine, FD C Blue 1, FD C Yellow 5.
| LENZAGEL
lidocaine hydrochloride, menthol gel |
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
| Labeler - Proficient Rx LP (079196022) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Proficient Rx LP | 079196022 | REPACK(63187-161) , RELABEL(63187-161) | |
Revised: 1/2021
Document Id: 6834737d-b0b4-46bd-8b10-a4b1b476e0f6
Set id: 7f7eedea-0723-4e8f-a250-4c786aeb95e8
Version: 3
Effective Time: 20210101
Proficient Rx LP