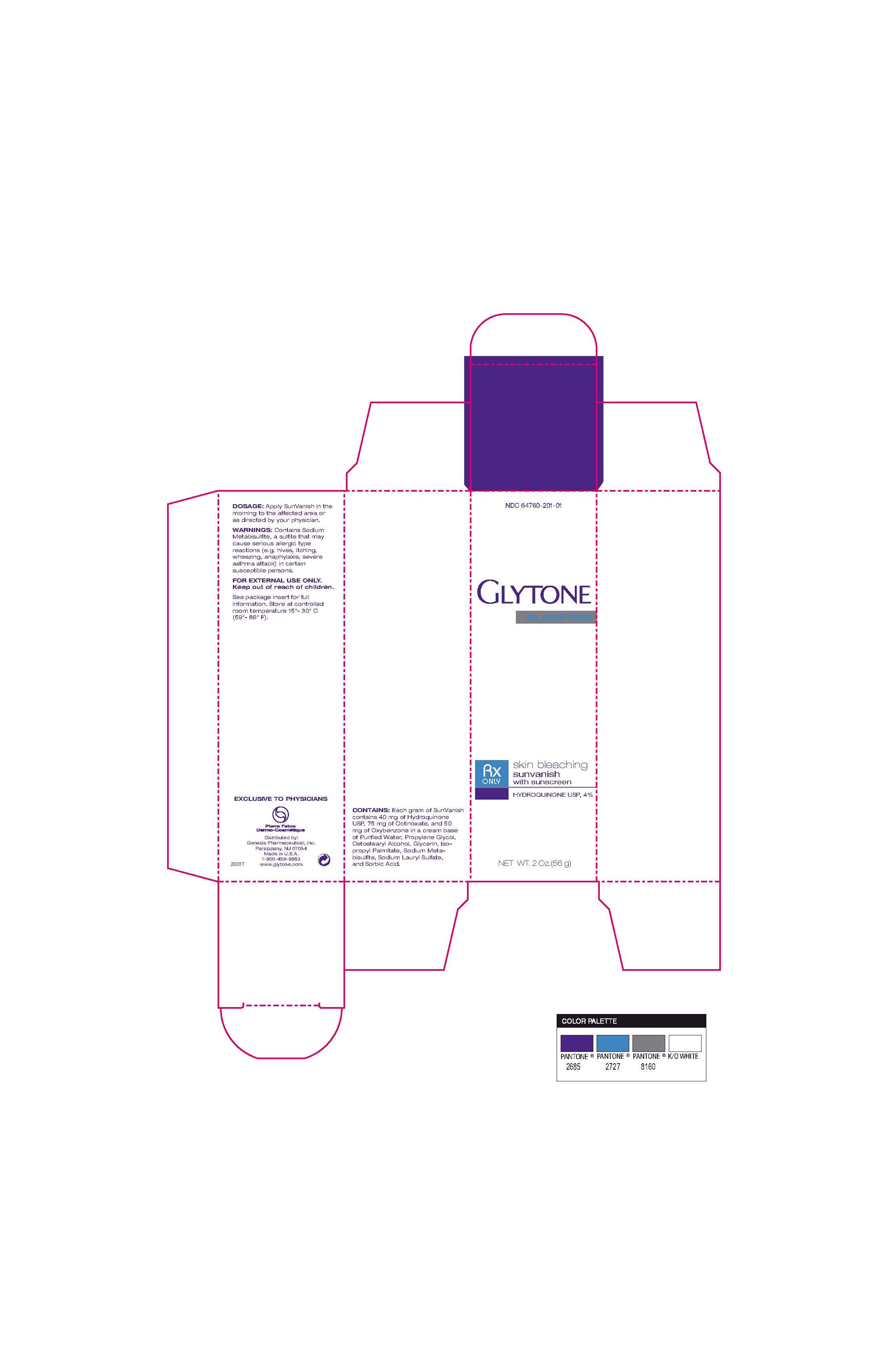
SUNVANISH- hydroquinone cream
Genesis Pharmaceutical, Inc.
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
----------
Glytone skin Bleaching sunvanish
Warnings Contains sodium Metabisulfite, a sulfite that may cause serious allergic type reactions (eg hives, itching, wheezing, anaphylaxis, severe asthma attack) in certain susceptible persons.
For external use only
Distributed by
Genesis Pharmaceuticals Inc
Parsippany, NJ 07054
Made in USA
18004598663
www.glytone.com
| SUNVANISH
hydroquinone cream |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Genesis Pharmaceutical, Inc. (117196928) |
Revised: 7/2014
Document Id: b57c1257-52a8-43bb-abd8-109734292f20
Set id: 7f285b34-6600-4fda-a58e-4bd157b68c75
Version: 4
Effective Time: 20140730
Genesis Pharmaceutical, Inc.