SINUS RELIEF PE MAXIMUM STRENGTH- guaifenesin, phenylephrine hcl tablet, film coated
L.N.K. International, Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Quality Plus 44-548
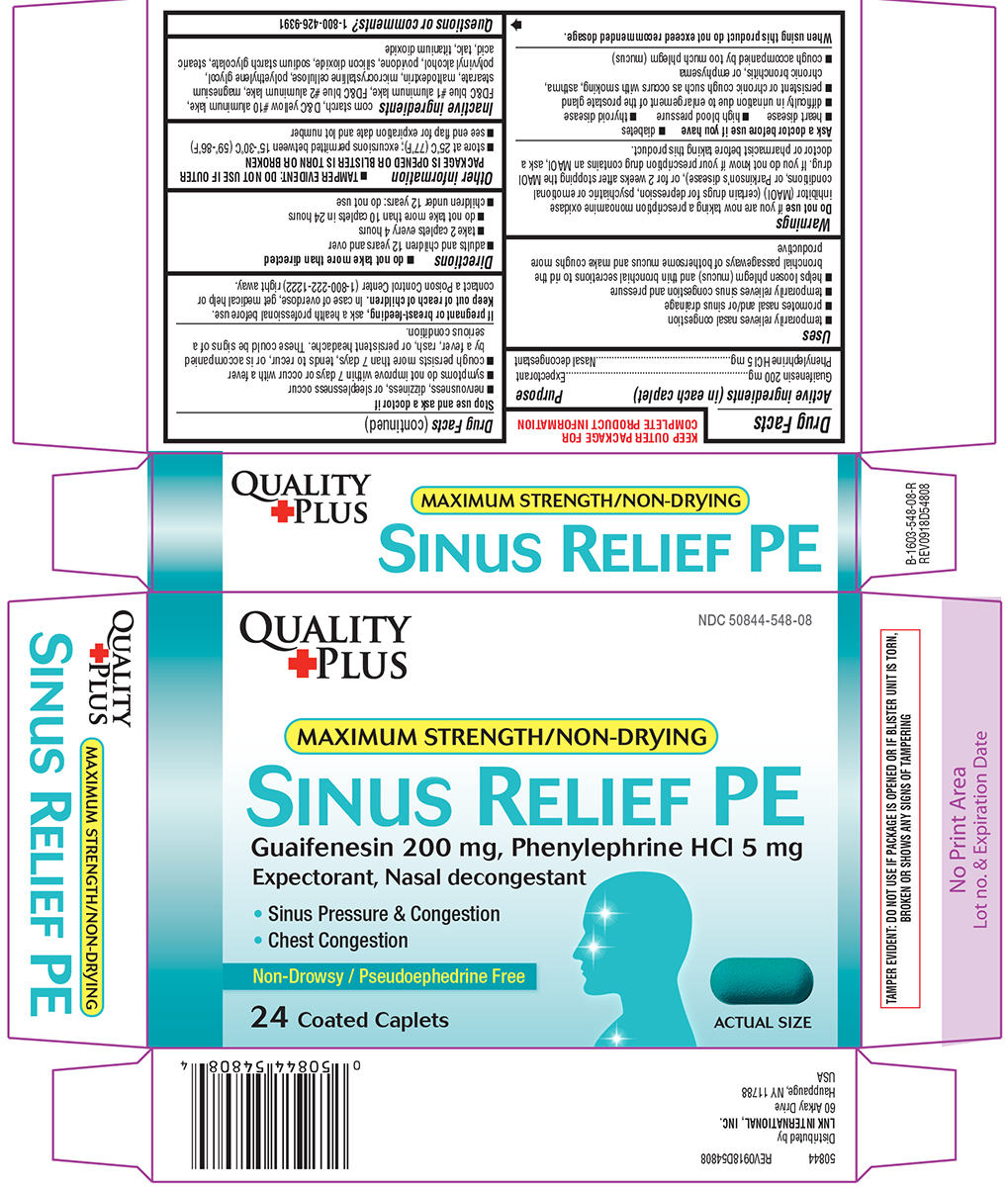
Uses
- temporarily relieves nasal congestion
- promotes nasal and/or sinus drainage
- temporarily relieves sinus congestion and pressure
- helps loosen phlegm (mucus) and thin bronchial secretions to rid the bronchial passageways of bothersome mucus and make coughs more productive
Warnings
Do not use
if you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric or emotional conditions, or Parkinson's disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product.
Ask a doctor before use if you have
- heart disease
- high blood pressure
- thyroid disease
- diabetes
- difficulty in urination due to enlargement of the prostate gland
- persistent or chronic cough such as occurs with smoking, asthma, chronic bronchitis, or emphysema
- cough accompanied by too much phlegm (mucus)
Directions
-
do not take more than directed
- adults and children 12 years and over
- take 2 caplets every 4 hours
- do not take more than 10 caplets in 24 hours
- take 2 caplets every 4 hours
- children under 12 years: do not use
Other information
- TAMPER EVIDENT: DO NOT USE IF OUTER PACKAGE IS OPENED OR BLISTER IS TORN OR BROKEN
- store at 25ºC (77ºF); excursions permitted between 15º-30ºC (59º-86ºF)
- see end flap for expiration date and lot number
Inactive ingredients
corn starch, D&C yellow #10 aluminum lake, FD&C blue #1 aluminum lake, FD&C blue #2 aluminum lake, magnesium stearate, maltodextrin, microcrystalline cellulose, polyethylene glycol, polyvinyl alcohol, povidone, silicon dioxide, sodium starch glycolate, stearic acid, talc, titanium dioxide
Principal Display Panel
QUALITY PLUS
NDC 50844-548-08
MAXIMUM STRENGTH / NON-DRYING
SINUS RELIEF PE
Guaifenesin 200 mg, Phenylephrine HCl 5 mg
Expectorant, Nasal decongestant
• Sinus Pressure & Congestion
• Chest Congestion
Non-Drowsy / Pseudoephedrine Free
24 Coated Caplets
Actual Size
TAMPER EVIDENT: DO NOT USE IF PACKAGE IS OPENED OR IF BLISTER UNIT IS TORN, BROKEN OR SHOWS ANY SIGNS OF TAMPERING
50844 REV0918D54808
Distributed by
LNK INTERNATIONAL, INC.
60 Arkay Drive
Hauppauge, NY 11788
USA
Quality Plus 44-586
| SINUS RELIEF PE
MAXIMUM STRENGTH
guaifenesin, phenylephrine hcl tablet, film coated |
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
| Labeler - L.N.K. International, Inc. (038154464) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| LNK International, Inc. | 832867894 | manufacture(50844-548) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| LNK International, Inc. | 832867837 | pack(50844-548) | |