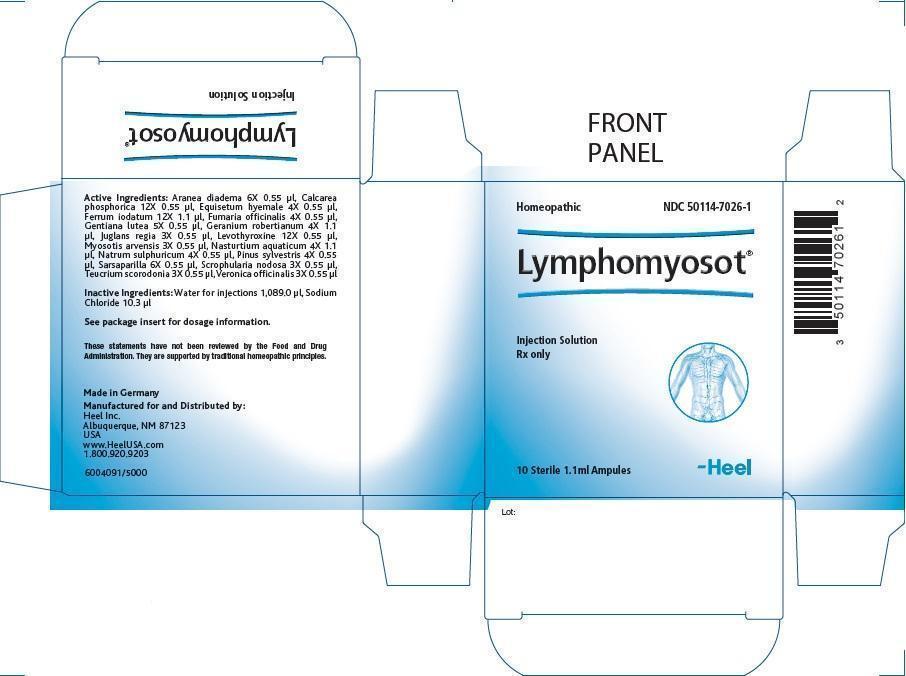
LYMPHOMYOSOT - geranium robertianum, nasturtium officinale, ferrous iodide, juglans regia leaf, juglans regia fruit rind, immature, myosotis arvensis, scrophularia nodosa, teucrium scorodonia flowering top, veronica officinalis flowering top, equisetum hyemale, fumaria officinalis flowering top, sodium sulfate, pinus sylvestris flowering top, gentiana lutea root, araneus diadematus, smilax regelii root, tribasic calcium phosphate and levothyroxine injection
Heel Inc
Disclaimer: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
----------
Lymphomyosot 1.1ml Injection
DESCRIPTION
| Each 1.1 ml ampule contains: | |||
| Active Ingredients: | |||
| Ingredient name | Potency | Quantity | Final dilution |
| Aranea diadema | 6X | 0.55 µl | 9.30X |
| Calcarea phosphorica | 12X | 0.55 µl | 15.30X |
| Equisetum hyemale | 4X | 0.55 µl | 7.30X |
| Ferrum iodatum | 12X | 1.1 µl | 15.00X |
| Fumaria officinalis | 4X | 0.55 µl | 7.30X |
| Gentiana lutea | 5X | 0.55 µl | 8.30X |
| Geranium robertianum | 4X | 1.1 µl | 7.00X |
| Juglans regia | 3X | 0.55 µl | 6.30X |
| Levothyroxine | 12X | 0.55 µl | 15.30X |
| Myosotis arvensis | 3X | 0.55 µl | 6.30X |
| Nasturtium aquaticum | 4X | 1.1 µl | 7.00X |
| Natrum sulphuricum | 4X | 0.55 µl | 7.30X |
| Pinus sylvestris | 4X | 0.55 µl | 7.30X |
| Sarsaparilla | 6X | 0.55 µl | 9.30X |
| Scrophularia nodosa | 3X | 0.55 µl | 6.30X |
| Teucrium scorodonia | 3X | 0.55 µl | 6.30X |
| Veronica officinalis | 3X | 0.55 µl | 6.30X |
Inactive Ingredients:
Water for injections 1,089.0 μl
Sodium Chloride 10.3 μl
INDICATION AND USAGE
Lymphomyosot® Injection Solution is a homeopathic drug product indicated for the improvement of lymphatic drainage, the non-specific immune defense, and conditions such as benign hypertrophy of lymphnodes, chronic tonsillitis, tonsillar hypertrophy and lymphatic edema.
DOSAGE AND ADMINISTRATION
General Considerations
- The dosage schedules listed below can be used as a general guide for the administration of Lymphomyosot® Injection Solution.
- Lymphomyosotl® Injection Solution may be administered s.c., i.d., i.m., or i.v.
- The interval between injections is left to the discretion of the HCP, but should not exceed 1 ampule in 24 hours.
- Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit.
- Draw up the contents of the ampule into the syringe.
- Discard any unused ampule contents. Do not reuse ampule.
Standard Dosage:
Adults and children 12 years and older: 1 ml 1 to 3 times per 7 days
Children 6 to 11 years: 0.7 ml 1 to 3 times per 7 days
Children 2 to 5 years: 0.5 ml 1 to 3 times per 7 days
Acute Dosage:
Adults and children 12 years and older: 1 ml daily, and then continue with standard dosage
Children 6 to 11 years: 0.7 ml daily, and then continue with standard dosage
Children 2 to 5 years: 0.5 ml daily, and then continue with standard dosage.
CONTRAINDICATIONS
Lymphomyosot® Injection Solution is contraindicated in patients with known hypersensitivity to Lymphomyosot® or any of its ingredients.
ADVERSE REACTIONS
Post-marketing Experience
- The following adverse events have been identified during post-marketing use of Lymphomyosot® Injection Solution. Because these events are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure
• Allergic (hypersensitivity) skin reactions may occur in isolated cases.
To report SUSPECTED ADVERSE REACTIONS, contact Heel Inc. at 1.800.920.9203
info@heelusa.com or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
OVERDOSAGE
No negative effects of an overdose have been reported and none are expected due to the homeopathic dilutions.
CLINICAL PHARMACOLOGY
Mechanism of Action
The exact mechanism of Lymphomyosot® Injection Solution is not fully understood.
Pharmacodynamics
Not applicable for homeopathic medicinal products.
| LYMPHOMYOSOT
geranium robertianum, nasturtium officinale, ferrous iodide, juglans regia leaf, juglans regia fruit rind, immature, myosotis arvensis, scrophularia nodosa, teucrium scorodonia flowering top, veronica officinalis flowering top, equisetum hyemale, fumaria officinalis flowering top, sodium sulfate, pinus sylvestris flowering top,gentiana lutea root, araneus diadematus, smilax regelii root, tribasic calcium phosphate and levothyroxine injection |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - Heel Inc (102783016) |