RELNATE DHA- omega-3 fatty acids, thiamine mononitrate, riboflavin, pyridoxine hydrochloride, cyanocobalamin, folic acid, cholecalciferol, ascorbic acid, alpha-tocopherol, cupric sulfate anhydrous, zinc oxide, iron, and magnesium capsule, liquid filled
Burel Pharmaceuticals, LLC
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
----------
RELNATE
DHA
PRENATAL / MULTIVITAMIN
Rev. 3/11
| Softgels | |
|---|---|
| Omega-3
Fatty Acids (DHA-EPA) | 200mg |
| Vitamin B1
(Thiamine Mononitrate) | 3 mg |
| Vitamin B2
(Riboflavin) | 3 mg |
| Vitamin B6
(Pyridoxine HCL) | 20 mg |
| Vitamin B12
(Cyanocobalamin) | 15 mcg |
| Folic Acid | 1 mg |
| Vitamin D3
(Cholecalciferol) | 400 IU |
| Vitamin C
(Ascorbic Acid) | 100 IU |
| Vitamin E
(d-alpha tocopherol) | 30 IU |
| Mineral Copper
(Sulfate) | 1 mg |
| Zinc
(Zinc Oxide) | 20 mg |
| Iron
(Fumerate) | 28 mg |
| Magnesium | 30 mg |
Rx ONLY
Description
Each Softgel Contains:
| Omega-3
Fatty Acids (DHA-EPA) | 200mg |
| Vitamin B1
(Thiamine Mononitrate) | 3 mg |
| Vitamin B2
(Riboflavin) | 3 mg |
| Vitamin B6
(Pyridoxine HCL) | 20 mg |
| Vitamin B12
(Cyanocobalamin) | 15 mcg |
| Folic Acid | 1 mg |
| Vitamin D3
(Cholecalciferol) | 400 IU |
| Vitamin C
(Ascorbic Acid) | 100 IU |
| Vitamin E
(d-alpha tocopherol) | 30 IU |
| Mineral Copper
(Sulfate) | 1 mg |
| Zinc
(Zinc Oxide) | 20 mg |
| Iron
(Fumerate) | 28 mg |
| Magnesium | 30 mg |
INDICATIONS AND USAGE
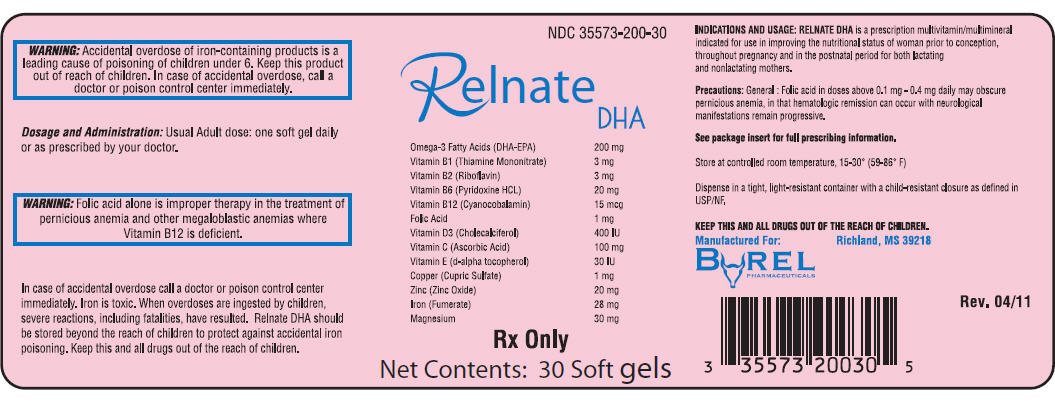
RELNATE DHA is a prescription multivitamin/multimineral indicated for use in improving the nutritional status of woman prior to conception, throughout pregnancy and in the postnatal period for both lactating and nonlactating mothers.
CONTRAINDICATIONS
RELNATE DHA should not be used by patients with known history of hypersensitivity to any of the listed ingredients.
PRECAUTIONS
Folic Acid alone is improper therapy in the treatment of pernicious anemia and other megaloblastic anemias where vitamin B12 is deficient. Folic Acid in doses above 0.1 mg daily may obscure pernicious anemia in that hematologic remission can occur while neurological manifestations progress. Allergic sensitization has been reported following both oral and parenteral administration of folic acid.
WARNING
Accidental overdose of iron-containing products is a leading cause of fatal poisoning in children under 6. KEEP THIS PRODUCT OUT OF REACH OF CHILDREN. In case of accidental overdose, call a doctor or poison control center immediately.
DRUG INTERACTIONS
RELNATE DHA softgels are not recommended for and should not be given to patients receiving levodopa because the action of levodopa is antagonized by pyridoxine.
ADVERSE REACTION
Adverse reactions have been reported with specific vitamins and minerals but generally at levels substantially higher than those contained herein. However, allergic and idiosyncratic reactions are possible at lower levels. Iron, even at the usual recommended levels, has been associated with gastrointestinal intolerance in some patients.
HOW SUPPLIED
RELNATE DHA is available as a Annato colored softgel, imprinted PRE 01. Available in 30 count bottles with NDC #35573-103-30 and sample cartons with NDC #35573-103-05.
NDC # 35573-103-05 PHYSICIAN SAMPLE NOT FOR RESALE
PRINCIPAL DISPLAY PANEL - 30 Soft gel Bottle Label
NDC 35573-200-30
Relnate
DHA
| Omega-3 Fatty Acids (DHA-EPA) | 200 mg |
| Vitamin B1 (Thiamine Mononitrate) | 3 mg |
| Vitamin B2 (Riboflavin) | 3 mg |
| Vitamin B6 (Pyridoxine HCL) | 20 mg |
| Vitamin B12 (Cyanocobalamin) | 15 mcg |
| Folic Acid | 1 mg |
| Vitamin D3 (Cholecalciferol) | 400 IU |
| Vitamin C (Ascorbic Acid) | 100 mg |
| Vitamin E (d-alpha tocopherol) | 30 IU |
| Copper (Cupric Sulfate) | 1 mg |
| Zinc (Zinc Oxide) | 20 mg |
| Iron (Fumerate) | 28 mg |
| Magnesium | 30 mg |
Rx Only
Net Contents: 30 Soft gels
| RELNATE DHA
omega-3 fatty acids, thiamine mononitrate, riboflavin, pyridoxine hydrochloride, cyanocobalamin, folic acid, cholecalciferol, ascorbic acid, alpha-tocopherol, cupric sulfate anhydrous, zinc oxide, iron, and magnesium capsule, liquid filled |
|||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||
| RELNATE DHA
omega-3 fatty acids, thiamine mononitrate, riboflavin, pyridoxine hydrochloride, cyanocobalamin, folic acid, cholecalciferol, ascorbic acid, alpha-tocopherol, cupric sulfate anhydrous, zinc oxide, iron, and magnesium capsule, liquid filled |
|||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||
| Labeler - Burel Pharmaceuticals, LLC (002152814) |
| Registrant - Burel Pharmaceuticals, Inc (002152814) |