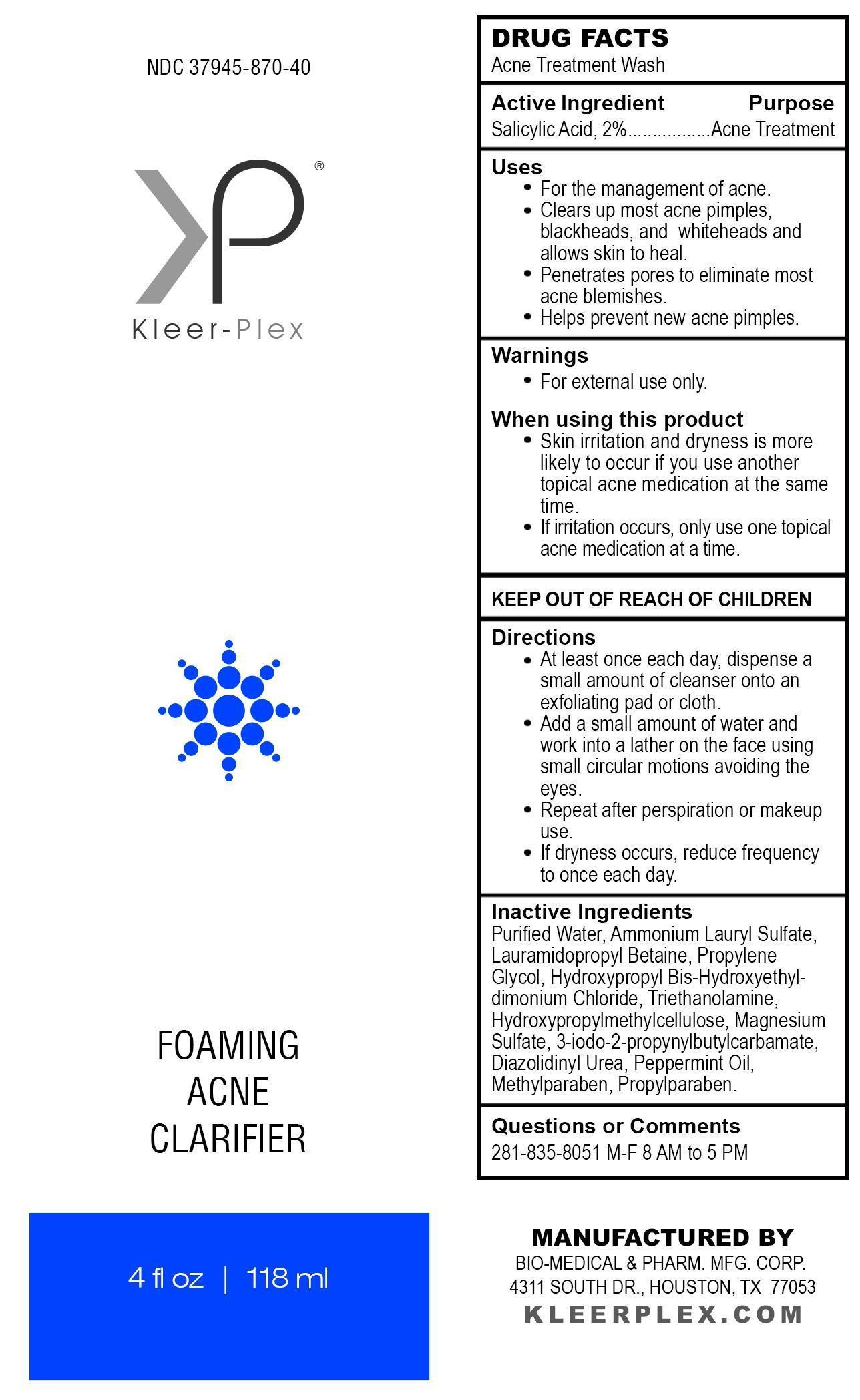
KLEER-PLEX FOAMING ACNE CLARIFIER- salicylic acid liquid
Bio-Medical & Pharmaceutical Manufacturing Corporation
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Use
For the management of acne.
Clears up most acne pimples, blackheads, and whiteheads and allows skin to heal.
Penetrates pores to eliminate most acne blemishes.
Helps prevent new acne pimples.
When using this product
Skin irritation and dryness is more likely to occur if you use another topical acne medication at the same time.
If irritation occurs, only use one topical acne medication at a time.
Directions
At least once each day, dispense a small amount of cleanser onto an exfoliating pad or cloth.
Add a small amount of water and work into a lather on the face using small circular motions avoiding the eyes.
Repeat after perspiration or makeup use.
If dryness occurs, reduce frequency to once each day.
Inactive Ingredients
Purified Water, Ammonium Lauryl Sulfate, Lauramidopropyl Betaine, Propylene Glycol, Hydroxypropyl Bis-Hydroxyethyldimonium Chloride, Triethanolamine, Hydroxypropylmethylcellulose, Magnesium Sulfate, 3-iodo-2-propynylbutylcarbamate, Diazolidinyl Urea, Peppermint Oil, Methylparaben, Propylparaben.
| KLEER-PLEX FOAMING ACNE CLARIFIER
salicylic acid liquid |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Labeler - Bio-Medical & Pharmaceutical Manufacturing Corporation (072186356) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Bio-Medical & Pharmaceutical Manufacturing Corporation | 072186356 | manufacture(37945-870) | |