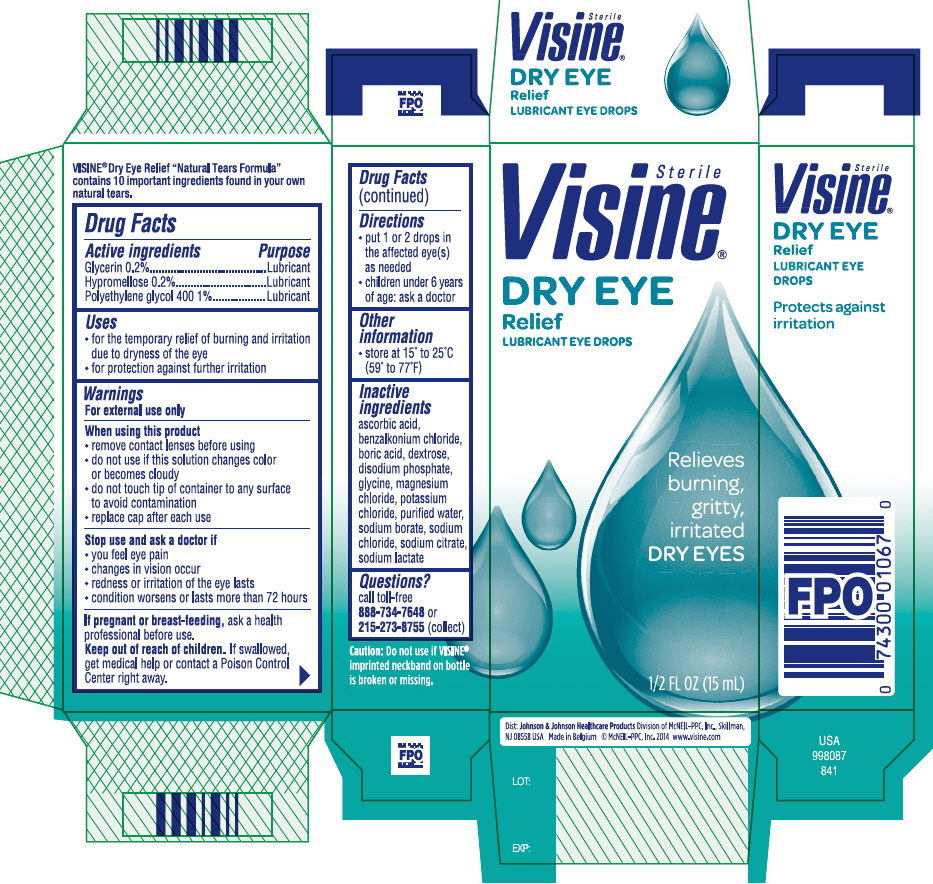
VISINE DRY EYE RELIEF- glycerin, hypromelloses, and polyethylene glycol 400 solution/ drops
Johnson & Johnson Consumer Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Visine® Dry Eye Relief Formula
Use
- for the temporary relief of burning and irritation due to dryness of the eye
- for protection against further irritation
Warnings
For external use only
When using this product
- remove contact lenses before using
- do not use if this solution changes color or becomes cloudy
- do not touch tip of container to any surface to avoid contamination
- replace cap after each use
Directions
- put 1 to 2 drops in the affected eye(s) as needed
- children under 6 years of age: ask a doctor
| VISINE DRY EYE RELIEF
glycerin, hypromelloses, and polyethylene glycol 400 solution/ drops |
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
| Labeler - Johnson & Johnson Consumer Inc. (002347102) |
Revised: 1/2020
Document Id: 9c9ebf9f-782d-4a6e-bb55-5e8b79a8992c
Set id: 7a34cecf-1fbc-4af7-894f-a4362f2eed58
Version: 4
Effective Time: 20200106
Johnson & Johnson Consumer Inc.