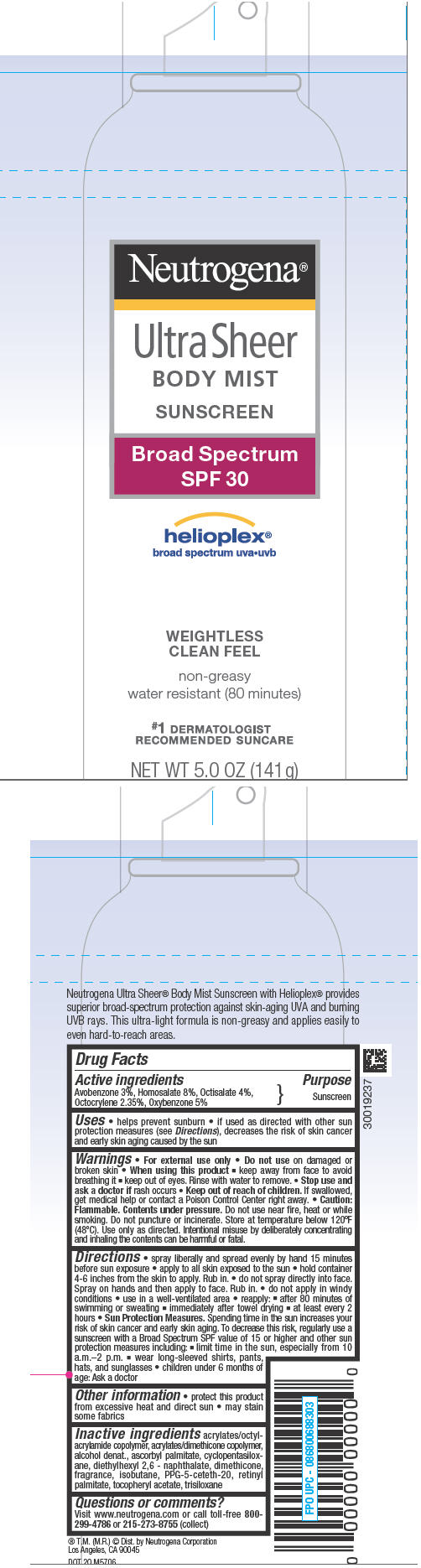
NEUTROGENA ULTRA SHEER BODY MIST SUNSCREEN BROAD SPECTRUM SPF 30- avobenzone, homosalate, octisalate, octocrylene, and oxybenzone aerosol, spray
Johnson & Johnson Consumer Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Ultra Sheer Body Mist Sunscreen
Broad Spectrum SPF 30
Uses
- Helps prevent sunburn
- If used as directed with other sun protection measures (see Directions), decreases the risk of skin cancer and early skin aging caused by the sun
Directions
- spray liberally and spread evenly by hand 15 minutes before sun exposure
- apply to all skin exposed to the sun
- hold container 4-6 inches from the skin to apply. Rub in
- do not spray directly into face. Spray on hands and then apply to face. Rub in.
- do not apply in windy conditions
- use in a well ventilated area
- reapply:
- after 80 minutes of swimming or sweating
- immediately after towel drying
- at least every 2 hours
-
Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a broad spectrum value of SPF of 15 or higher and other sun protection measures including:
- Limit time in the sun, especially from 10 a.m. – 2 p.m.
- Wear long-sleeved shirts, pants hats, and sunglasses
- Children under 6 months of age: Ask a doctor
Inactive Ingredients
Acrylates/ Octylacrylamide Copolymer, Acrylates/Dimethicone Copolymer, Alcohol Denat. , Ascorbyl Palmitate, Cyclopentasiloxane, Diethylhexyl 2,6 – Naphthalate, Dimethicone , Fragrance, Isobutane, PPG-5-Ceteth-20, Retinyl Palmitate, Tocopheryl Acetate, Trisiloxane
| NEUTROGENA ULTRA SHEER BODY MIST SUNSCREEN
BROAD SPECTRUM SPF 30
avobenzone, homosalate, octisalate, octocrylene, and oxybenzone aerosol, spray |
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
| Labeler - Johnson & Johnson Consumer Inc. (002347102) |
Revised: 8/2015
Document Id: d4510a36-5058-4610-9300-9bb77fc314dc
Set id: 7915955b-3c76-41d0-b218-29b13afb2c04
Version: 2
Effective Time: 20150803
Johnson & Johnson Consumer Inc.