Label: CLAVAMOX- amoxicillin and clavulanate potassium tablet, chewable
-
NDC Code(s):
54771-1020-1,
54771-1020-2,
54771-1021-1,
54771-1021-2, view more54771-1022-1, 54771-1022-2, 54771-1023-1, 54771-1023-2
- Packager: Zoetis Inc.
- Category: PRESCRIPTION ANIMAL DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Animal Drug Application
Drug Label Information
Updated September 25, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- CAUTION
-
DESCRIPTION
CLAVAMOX CHEWABLE Tablet (amoxicillin and clavulanate potassium tablets) is an orally administered formulation comprised of the broad-spectrum antibiotic Amoxi® (amoxicillin trihydrate) and the β-lactamase inhibitor, clavulanate potassium (the potassium salt of clavulanic acid).
Amoxicillin trihydrate is a semisynthetic antibiotic with a broad spectrum of bactericidal activity against many gram-positive and gram-negative, aerobic and anaerobic microorganisms. It does not resist destruction by β-lactamases; therefore, it is not effective against β-lactamase-producing bacteria. Chemically, it is D(-)-α-amino-p-hydroxybenzyl penicillin trihydrate.
Clavulanic acid, an inhibitor of β-lactamase enzymes, is produced by the fermentation of Streptomyces clavuligerus. Clavulanic acid by itself has only weak antibacterial activity. Chemically, clavulanate potassium is potassium z-(3R,5R)-2-β-hydroxyethylidene clavam-3-carboxylate.
-
INDICATIONS
CLAVAMOX CHEWABLE Tablets are indicated in the treatment of:
Dogs: Skin and soft tissue infections such as wounds, abscesses, cellulitis, superficial/juvenile and deep pyoderma due to susceptible strains of the following organisms: β-lactamase-producing Staphylococcus aureus, non-β-lactamase-producing Staphylococcus aureus, Staphylococcus spp., Streptococcus spp., and E. coli.
Periodontal infections due to susceptible strains of both aerobic and anaerobic bacteria. CLAVAMOX CHEWABLE has been shown to be clinically effective for treating cases of canine periodontal disease.
Cats: Skin and soft tissue infections such as wounds, abscesses, and cellulitis/dermatitis due to susceptible strains of the following organisms: β-lactamase-producing Staphylococcus aureus, non-β-lactamase-producing Staphylococcus aureus, Staphylococcus spp., Streptococcus spp., E. coli, and Pasteurella spp.
Urinary tract infections (cystitis) due to susceptible strains of E. coli.
Therapy may be initiated with CLAVAMOX CHEWABLE prior to obtaining results from bacteriological and susceptibility studies. A culture should be obtained prior to treatment to determine susceptibility of the organisms to CLAVAMOX. Following determination of susceptibility results and clinical response to medication, therapy may be reevaluated.
-
DOSAGE AND ADMINISTRATION:
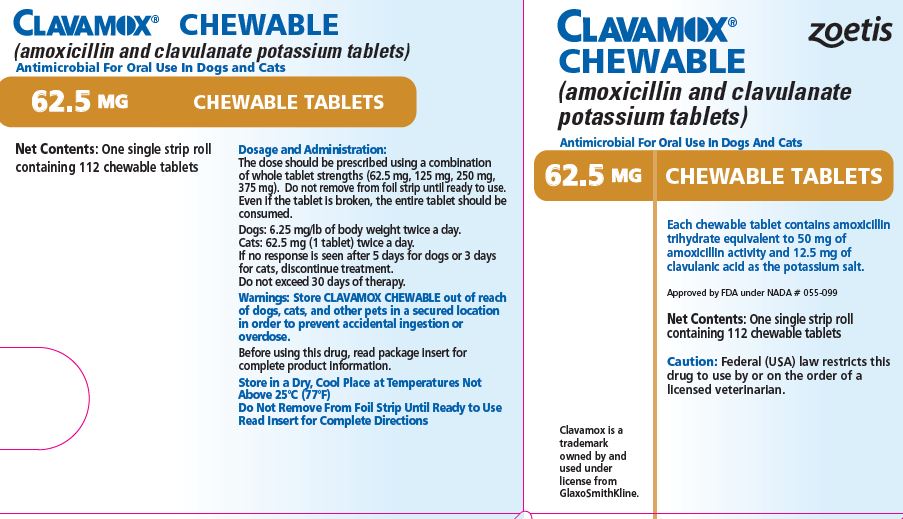
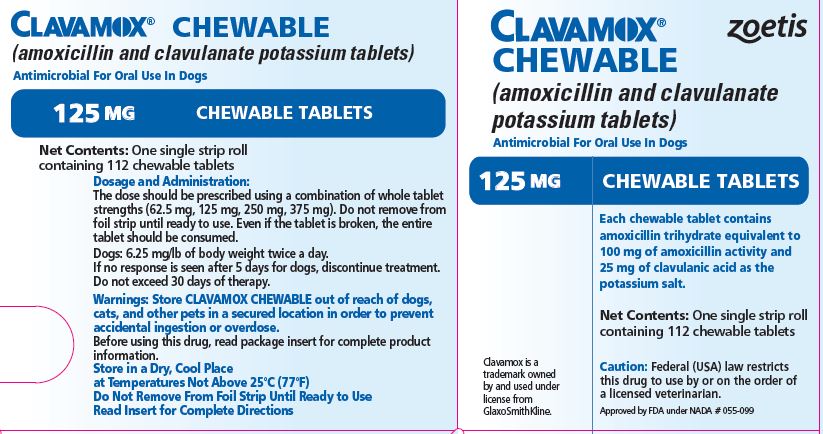
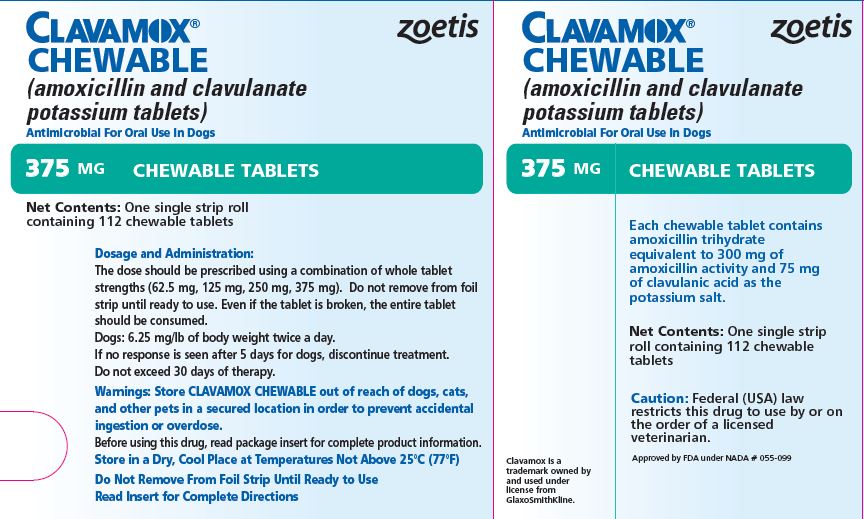
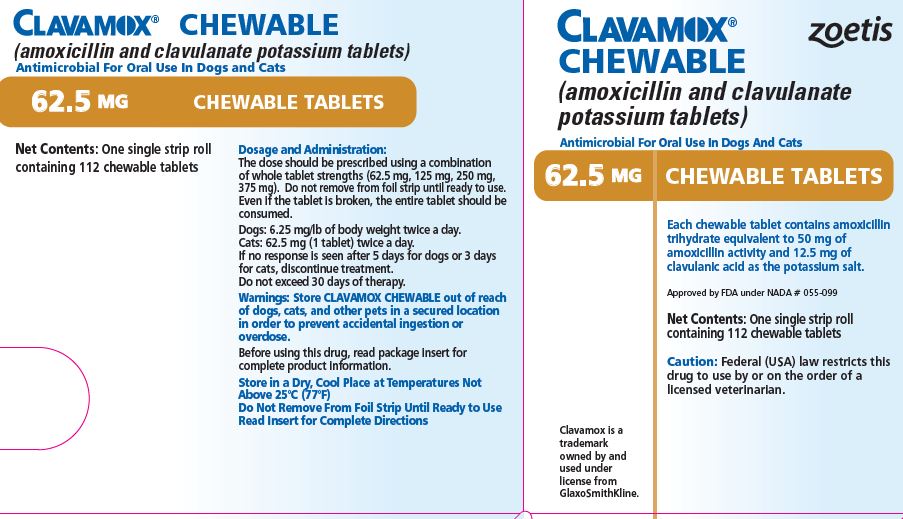
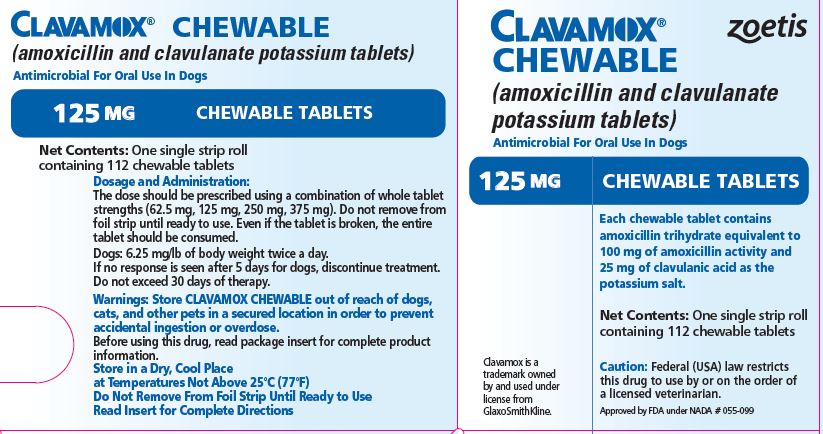
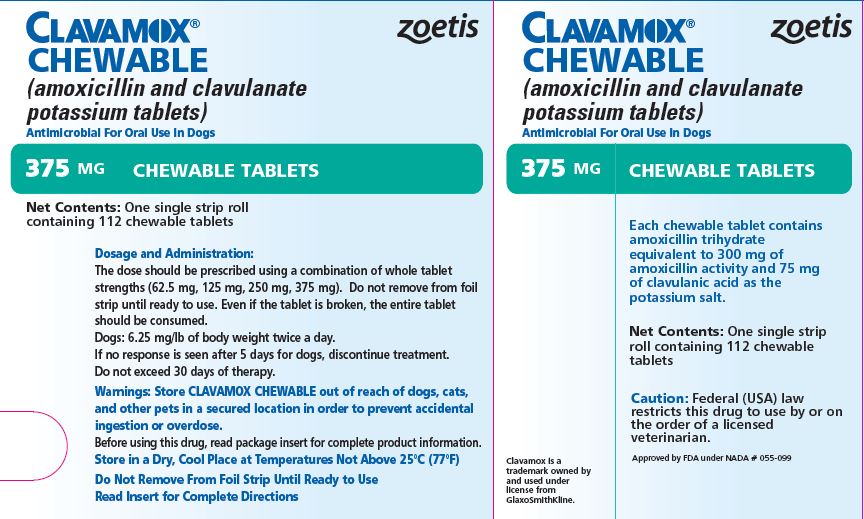
The dose should be prescribed using a combination of whole tablet strengths (62.5 mg, 125 mg, 250 mg, 375 mg). Do not remove from foil strip until ready to use. Even if the tablet is broken, the entire tablet should be consumed.
Dogs
The recommended dosage of CLAVAMOX CHEWABLE Tablet is 6.25 mg/lb of body weight twice a day.
Skin and soft tissue infections such as abscesses, cellulitis, wounds, superficial/juvenile pyoderma, and periodontal infections should be treated for 5-7 days or for 48 hours after all symptoms have subsided.
If no response is seen after 5 days of treatment, therapy should be discontinued and the case reevaluated. Deep pyoderma may require treatment for 21 days; the maximum duration of treatment should not exceed 30 days.
Cats
The recommended dosage of CLAVAMOX CHEWABLE Tablet is 62.5 mg twice a day.
Skin and soft tissue infections such as abscesses and cellulitis/dermatitis should be treated for 5-7 days or for 48 hours after all symptoms have subsided, not to exceed 30 days. If no response is seen after 3 days of treatment, therapy should be discontinued and the case reevaluated.
Urinary tract infections may require treatment for 10-14 days or longer. The maximum duration of treatment should not exceed 30 days.
- CONTRAINDICATIONS
-
WARNINGS
Store CLAVAMOX CHEWABLE out of reach of dogs, cats, and other pets in a secured location in order to prevent accidental ingestion or overdose.
HUMAN WARNINGS: Not for human use. Keep this and all drugs out of reach of children. Antimicrobial drugs, including penicillins and cephalosporins, can cause allergic reactions in sensitized individuals.
To minimize the possibility of allergic reactions, those handling such antimicrobials, including amoxicillin trihydrate/clavulanate potassium, are advised to avoid direct contact of the product with the skin and mucous membranes.
-
PRECAUTIONS
Prescribing antibacterial drugs in the absence of a proven or strongly suspected bacterial infection is unlikely to provide benefit to treated animals and may increase the risk of the development of drug-resistant animal pathogens. Safety of use in pregnant or breeding animals has not been determined.
-
ADVERSE REACTIONS
CLAVAMOX CHEWABLE contains a semisynthetic penicillin (amoxicillin) and has the potential for producing allergic reactions. If an allergic reaction occurs, administer epinephrine and/or steroids.
Post-Approval Experience (July, 2017):
The following adverse events are based on post-approval adverse drug experience reporting. Not all adverse events are reported to FDA/CVM. It is not always possible to reliably estimate the adverse event frequency or establish a causal relationship to product exposure using these data. The following adverse events reported for dogs and cats are listed in decreasing order of reporting frequency for CLAVAMOX: Anorexia, lethargy, vomiting and diarrhea.
For a copy of the Safety Data Sheet or to report adverse reactions, call Zoetis Inc. at 1-888-963-8471. For additional information about adverse drug experience reporting for animal drugs, contact FDA at 1-888-FDA-VETS or www.fda.gov/reportanimalae. -
ACTIONS
The 2 components are rapidly absorbed resulting in amoxicillin and clavulanic acid concentrations in serum, urine, and tissues similar to those produced when each is administered alone.
Amoxicillin and clavulanic acid diffuse readily into most body tissues and fluids with the exception of brain and spinal fluid, which amoxicillin penetrates adequately when meninges are inflamed. Most of the amoxicillin is excreted unchanged in the urine. Clavulanic acid’s penetration into spinal fluid is unknown at this time. Approximately 15% of the administered dose of clavulanic acid is excreted in the urine within the first 6 hours.
CLAVAMOX CHEWABLE combines the distinctive properties of a broad-spectrum antibiotic and a β-lactamase inhibitor to effectively extend the antibacterial spectrum of amoxicillin to include β-lactamase-producing as well as non-β-lactamase-producing aerobic and anaerobic organisms. -
MICROBIOLOGY
Amoxicillin is bactericidal in action and acts through the inhibition of biosynthesis of cell wall mucopeptide of susceptible organisms. The action of clavulanic acid extends the antimicrobial spectrum of amoxicillin to include organisms resistant to amoxicillin and other β-lactam antibiotics. Amoxicillin/clavulanate has been shown to have a wide range of activity which includes β-lactamase-producing strains of both gram-positive and gram-negative aerobes, facultative anaerobes, and obligate anaerobes. Many strains of the following organisms, including β-lactamase-producing strains, isolated from veterinary sources, were found to be susceptible to amoxicillin/ clavulanate in vitro but the clinical significance of this activity has not been demonstrated for some of these organisms in animals.
Aerobic bacteria, including Staphylococcus aureus*, β-lactamase-producing Staphylococcus aureus* (penicillin resistant), Staphylococcus species*, Staphylococcus epidermidis, Staphylococcus intermedius, Streptococcus faecalis, Streptococcus species*, Corynebacterium pyogenes, Corynebacterium species, Erysipelothrix rhusiopathiae, Bordetella bronchiseptica, Escherichia coli*, Proteus mirabilis, Proteus species, Enterobacter species, Klebsiella pneumoniae, Salmonella dublin, Salmonella typhimurium, Pasteurella multocida, Pasteurella hemolytica, Pasteurella species*.
* The susceptibility of these organisms has also been demonstrated in in vivo studies.
Studies have demonstrated that both aerobic and anaerobic flora are isolated from gingival cultures of dogs with clinical evidence of periodontal disease. Both gram-positive and gram-negative aerobic and anaerobic subgingival isolates indicate sensitivity to amoxicillin/ clavulanic acid during antimicrobial susceptibility testing.
-
SUSCEPTIBILITY TEST
The recommended quantitative disc susceptibility method (FEDERAL REGISTER 37:20527-29; Bauer AW, Kirby WMM, Sherris JC, et al: Antibiotic susceptibility testing by standardized single disc method. Am J Clin Path 45:493, 1966) utilized 30 mcg Augmentin® (AMC) discs for estimating the susceptibility of bacteria to CLAVAMOX CHEWABLE Tablets.
-
PALATABILITY
The palatability of CLAVAMOX CHEWABLE Tablets was evaluated in a multi-location field trial. One hundred twelve (112) client-owned dogs were dosed with CLAVAMOX CHEWABLE Tablets at 6.25 mg/lb (12.5 mg/kg) twice daily for 7 days and evaluated for palatability of the product. Dogs freely consumed 83% of their doses within 5 minutes of offering from an empty bowl or owner’s hand. Of the 17% of doses unconsumed after 5 minutes, 16% were administered with a treat/food or forced intake and 1% of doses were refused.
- STORAGE INFORMATION
-
HOW SUPPLIED
CLAVAMOX CHEWABLE Tablets in the following strengths are supplied in single strip rolls containing 112 chewable tablets.
Each 62.5-mg tablet contains amoxicillin trihydrate equivalent to 50 mg of amoxicillin activity and 12.5 mg of clavulanic acid as the potassium salt. For use in dogs and cats.
Each 125-mg tablet contains amoxicillin trihydrate equivalent to 100 mg of amoxicillin activity and 25 mg of clavulanic acid as the potassium salt. For use in dogs only.
Each 250-mg tablet contains amoxicillin trihydrate equivalent to 200 mg of amoxicillin activity and 50 mg of clavulanic acid as the potassium salt. For use in dogs only.
Each 375-mg tablet contains amoxicillin trihydrate equivalent to 300 mg of amoxicillin activity and 75 mg of clavulanic acid as the potassium salt. For use in dogs only.
Dispense according to recommendations outlined in Dosage and Administration section.
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 62.5 mg Tablet Carton
- PRINCIPAL DISPLAY PANEL - 125 mg Tablet Carton
- PRINCIPAL DISPLAY PANEL - 250 mg Tablet Carton
- PRINCIPAL DISPLAY PANEL - 375 mg Tablet Carton
-
INGREDIENTS AND APPEARANCE
CLAVAMOX
amoxicillin and clavulanate potassium tablet, chewableProduct Information Product Type PRESCRIPTION ANIMAL DRUG Item Code (Source) NDC:54771-1023 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength AMOXICILLIN (UNII: 804826J2HU) (AMOXICILLIN ANHYDROUS - UNII:9EM05410Q9) AMOXICILLIN ANHYDROUS 50 mg CLAVULANATE POTASSIUM (UNII: Q42OMW3AT8) (CLAVULANIC ACID - UNII:23521W1S24) CLAVULANIC ACID 12.5 mg Product Characteristics Color brown (mottled) Score 2 pieces Shape SQUARE Size 9mm Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:54771-1023-1 10 in 1 CARTON 1 10 in 1 BLISTER PACK 2 NDC:54771-1023-2 1 in 1 CARTON 2 112 in 1 BLISTER PACK Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NADA NADA055099 05/24/2017 CLAVAMOX
amoxicillin and clavulanate potassium tablet, chewableProduct Information Product Type PRESCRIPTION ANIMAL DRUG Item Code (Source) NDC:54771-1020 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength AMOXICILLIN (UNII: 804826J2HU) (AMOXICILLIN ANHYDROUS - UNII:9EM05410Q9) AMOXICILLIN ANHYDROUS 100 mg CLAVULANATE POTASSIUM (UNII: Q42OMW3AT8) (CLAVULANIC ACID - UNII:23521W1S24) CLAVULANIC ACID 25 mg Product Characteristics Color brown (mottled) Score 2 pieces Shape SQUARE Size 11mm Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:54771-1020-2 1 in 1 CARTON 1 112 in 1 BLISTER PACK 2 NDC:54771-1020-1 10 in 1 CARTON 2 10 in 1 BLISTER PACK Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NADA NADA055099 05/24/2017 CLAVAMOX
amoxicillin and clavulanate potassium tablet, chewableProduct Information Product Type PRESCRIPTION ANIMAL DRUG Item Code (Source) NDC:54771-1021 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength AMOXICILLIN (UNII: 804826J2HU) (AMOXICILLIN ANHYDROUS - UNII:9EM05410Q9) AMOXICILLIN ANHYDROUS 200 mg CLAVULANATE POTASSIUM (UNII: Q42OMW3AT8) (CLAVULANIC ACID - UNII:23521W1S24) CLAVULANIC ACID 50 mg Product Characteristics Color brown (mottled) Score 2 pieces Shape SQUARE Size 15mm Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:54771-1021-1 10 in 1 CARTON 1 10 in 1 BLISTER PACK 2 NDC:54771-1021-2 1 in 1 CARTON 2 112 in 1 BLISTER PACK Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NADA NADA055099 05/24/2017 CLAVAMOX
amoxicillin and clavulanate potassium tablet, chewableProduct Information Product Type PRESCRIPTION ANIMAL DRUG Item Code (Source) NDC:54771-1022 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength AMOXICILLIN (UNII: 804826J2HU) (AMOXICILLIN ANHYDROUS - UNII:9EM05410Q9) AMOXICILLIN ANHYDROUS 300 mg CLAVULANATE POTASSIUM (UNII: Q42OMW3AT8) (CLAVULANIC ACID - UNII:23521W1S24) CLAVULANIC ACID 75 mg Product Characteristics Color brown (mottled) Score 2 pieces Shape SQUARE Size 18mm Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:54771-1022-2 1 in 1 CARTON 1 112 in 1 BLISTER PACK 2 NDC:54771-1022-1 10 in 1 CARTON 2 10 in 1 BLISTER PACK Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NADA NADA055099 05/24/2017 Labeler - Zoetis Inc. (828851555)