CETYLEV- acetylcysteine tablet, effervescent
Arbor Pharmaceuticals
----------
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use CETYLEV® safely and effectively. See full prescribing information for CETYLEV.
CETYLEV (acetylcysteine) effervescent tablets for oral solution Initial U.S. Approval: 1963 INDICATIONS AND USAGECETYLEV is an antidote for acetaminophen overdose indicated to prevent or lessen hepatic injury after ingestion of a potentially hepatotoxic quantity of acetaminophen in patients with acute ingestion or from repeated supratherapeutic ingestion. (1) DOSAGE AND ADMINISTRATIONPre-Treatment Assessment Following Acute Ingestion (2.1): Obtain a plasma or serum sample to assay for acetaminophen concentration at least 4 hours after ingestion.
Nomogram for Estimating Potential for Hepatotoxicity from Acute Acetaminophen Ingestion (2.2):
Recommended Adult and Pediatric Dosage (2.3):
Repeated Supratherapeutic Acetaminophen Ingestion (2.4):
DOSAGE FORMS AND STRENGTHSEffervescent tablets: 500 mg and 2.5 grams (3) CONTRAINDICATIONSNone (4) WARNINGS AND PRECAUTIONS
ADVERSE REACTIONSMost common adverse reactions are nausea and vomiting, other gastrointestinal symptoms, and rash with or without fever. (6) To report SUSPECTED ADVERSE REACTIONS, contact Arbor Pharmaceuticals LLC at 1- 866-516-4950 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch. See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling. Revised: 4/2017 |
FULL PRESCRIBING INFORMATION
1 INDICATIONS AND USAGE
CETYLEV is indicated to prevent or lessen hepatic injury after ingestion of a potentially hepatotoxic quantity of acetaminophen in patients with acute ingestion or from repeated supratherapeutic ingestion (RSI).
2 DOSAGE AND ADMINISTRATION
2.1 Pretreatment Assessment and Testing Following Acute Acetaminophen Ingestion
The following recommendations are related to acute acetaminophen ingestion. For recommendations related to repeated supratherapeutic exposure see Dosage and Administration (2.4).
- Assess the history and timing of acetaminophen ingestion as an overdose.
- The reported history of the quantity of acetaminophen ingested as an overdose is often inaccurate and is not a reliable guide to therapy.
- Obtain the following laboratory tests to monitor hepatic and renal function and electrolyte and fluid balance: aspartate aminotransferase (AST), alanine aminotransferase (ALT), bilirubin, international normalized ratio (INR), creatinine, blood urea nitrogen (BUN), blood glucose, and electrolytes.
- Obtain a plasma or serum sample to assay for acetaminophen concentration at least 4 hours after ingestion. Acetaminophen concentrations obtained earlier than 4 hours post-ingestion may be misleading as they may not represent maximum acetaminophen concentrations.
- If the time of acute acetaminophen ingestion is unknown:
- Administer a loading dose of CETYLEV immediately [see Dosage and Administration (2.3, 2.4)].
- Obtain an acetaminophen concentration to determine need for continued treatment [see Dosage and Administration (2.2)].
- If the acetaminophen concentration cannot be obtained (or is unavailable or uninterpretable) within the 8-hour time interval after acetaminophen ingestion or there is clinical evidence of acetaminophen toxicity:
- Administer a loading dose of CETYLEV immediately and continue treatment for a total of 17 doses [see Dosage and Administration (2.3)].
- If the patient presents more than 8 hours after ingestion and the time of acute acetaminophen ingestion is known:
- Administer a loading dose of CETYLEV immediately [see Dosage and Administration (2.3)].
- Obtain acetaminophen concentration to determine need for continued treatment [see Dosage and Administration (2.2)].
- If the patient presents less than 8 hours after ingestion and the time of acute acetaminophen ingestion is known and the acetaminophen concentration is known:
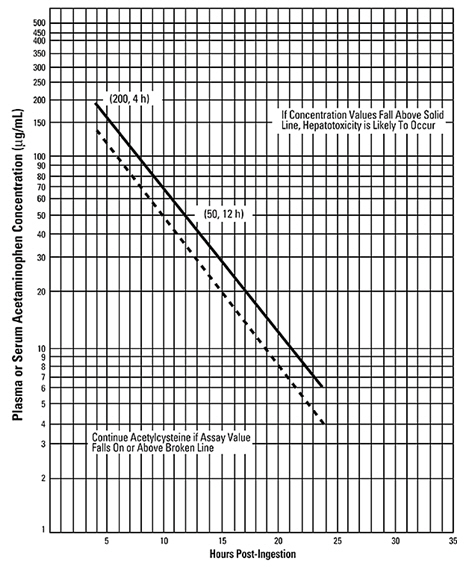
- Use the Rumack-Matthew nomogram (Figure 1) to determine whether or not to initiate treatment with CETYLEV [see Dosage and Administration (2.2)].
2.2 Nomogram for Estimating Potential for Hepatotoxicity from Acute Acetaminophen Ingestion and Need for CETYLEV Treatment
If the timing of the acute acetaminophen ingestion is known and the results of the acetaminophen assay are available within 8 hours:
- Refer to the Rumack-Matthew nomogram (see Figure 1) to determine whether or not to initiate treatment with CETYLEV.
- Initiation of CETYLEV depends on the acetaminophen concentration and also the clinical presentation of the patient.
The nomogram may underestimate the hepatotoxicity risk in patients with chronic alcoholism, malnutrition, or CYP2E1 enzyme inducing drugs (e.g., isoniazid), and consideration should be given to treating these patients even if the acetaminophen concentrations are in the nontoxic range.
Loading Dose
For patients whose acetaminophen concentrations are at or above the "possible" toxicity line (dotted line in nomogram):
- Administer a loading dose of CETYLEV [see Dosage and Administration (2.3)].
For patients with an acute overdose due to an extended-release acetaminophen, if the acetaminophen concentration at 4 hours post ingestion is below the possible toxicity line then obtain a second sample for acetaminophen concentration 8 to 10 hours after the acute ingestion. If the second value is at or above the "possible" toxicity line (dotted line in nomogram):
- Administer a loading dose of CETYLEV [see Dosage and Administration (2.3)].
For patients whose values are below the "possible" toxicity line, but time of ingestion was unknown or sample was obtained less than 4 hours after ingestion:
- Administer a loading dose of CETYLEV [see Dosage and Administration (2.3)].
For patients whose values are below the "possible" toxicity line and time of ingestion is known and the sample was obtained more than 4 hours after ingestion, do not administer CETYLEV because there is minimal risk of hepatotoxicity.
| Figure 1: Rumack-Matthew Nomogram for Estimating Potential for Hepatotoxicity from Acetaminophen Poisoning – Plasma or Serum Acetaminophen Concentration versus Time (hours) Post-acetaminophen Ingestion (Adapted from Rumack and Matthew, Pediatrics 1975; 55:871−876.) | |
 |
|
Maintenance Dose
Determine need for continued treatment with CETYLEV after the loading dose. Choose ONE of the following based on the acetaminophen concentration:
The acetaminophen concentration is above the possible toxicity line according to the nomogram (see Figure 1):
- Continue CETYLEV treatment with the maintenance dose for 17 doses [see Dosage and Administration (2.3)].
- Monitor hepatic and renal function and electrolytes throughout treatment.
The acetaminophen concentration could not be obtained:
- Continue CETYLEV treatment with the maintenance dose for 17 doses [see Dosage and Administration (2.3)].
- Monitor hepatic and renal function and electrolytes throughout treatment.
For patients whose acetaminophen concentration is below the "possible" toxicity line (see Figure 1) and time of ingestion is known and the sample was obtained more than 4 hours after ingestion:
- Discontinue CETYLEV.
The acetaminophen concentration was in the non-toxic range, but time of ingestion was unknown or less than 4 hours:
- Obtain a second sample for acetaminophen concentration and consider the patient's clinical status to decide whether or not to continue CETYLEV treatment.
- If there is any uncertainty as to patient's risk of developing hepatotoxicity, it is recommended to administer a complete treatment course under medical observation with appropriate monitoring.
Continued Therapy After Completion of Loading and Maintenance Doses
In cases of suspected massive overdose, or with concomitant ingestion of other substances, or in patients with preexisting liver disease; the absorption and/or the half-life of acetaminophen may be prolonged. In such cases, consideration should be given to the need for continued treatment with CETYLEV beyond a total of 17 maintenance doses.
Acetaminophen levels and ALT/AST and INR should be checked after the last maintenance dose. If acetaminophen levels are still detectable, or if the ALT/AST are still increasing or the INR remains elevated; the maintenance doses should be continued and the treating physician should contact a US regional poison center at 1-800-222-1222, or alternatively, a "special health professional assistance line for acetaminophen overdose" at 1-800-525-6115 for assistance with dosing recommendations.
2.3 Recommended Dosage and Preparation and Administration Instructions in Adults and Pediatrics for Acute Acetaminophen Ingestion
- CETYLEV is for oral administration only; not for nebulization or intratracheal instillation.
- After appropriate preparation and dilution, CETYLEV is interchangeable with 20% acetylcysteine solution, when given at the same acetylcysteine dosage.
- Adults and Pediatrics: The recommended loading dose of CETYLEV is 140 mg/kg. Administer a first maintenance dose of 70 mg/kg 4 hours after the loading dose. Repeat 70 mg/kg maintenance dose every 4 hours for a total of 17 maintenance doses.
Preparation and Administration Instructions
- Dissolve the appropriate number of 2.5 gram and/or 500 mg CETYLEV effervescent tablets in the volume of water indicated in dosing tables and text below, based upon patient weight.
- Once the tablets are dissolved, administer the oral solution immediately.
- Solutions should be freshly prepared for each dose and utilized within 2 hours.
- If the patient vomits an oral dose of CETYLEV within 1 hour of administration, repeat that dose.
- If the patient is persistently unable to retain the orally administered acetylcysteine, CETYLEV may be administered by nasoduodenal tube. An intravenous formulation of acetylcysteine may also be considered.
Patients Weighing 20 kg and Greater
Tables 1 and 2 provide the weight-based loading and maintenance doses, respectively, of CETYLEV for patients weighing 20 kg and greater. For patients weighing 20 to 59 kg dissolve CETYLEV tablets in 150 mL of water. For patients weighing 60 kg and greater dissolve CETYLEV tablets in 300 mL of water.
| *No specific studies have been conducted to evaluate the necessity of dose adjustments in patients weighing over 100 kg. Limited information is available regarding the dosing requirements of patients that weigh more than 100 kg. | |||
| Dissolve CETYLEV Tablets in 300 mL of Water | |||
| Body weight (Kg) | Actual Acetylcysteine Dose to be Administered (grams) | Number of CETYLEV Tablets to Dissolve in Water | |
| 2.5 gram tablets | 500 mg tablets | ||
| 100 or greater | 15 | 6 | 0 |
| 90 to 99 | 14 | 5 | 3 |
| 80 to 89 | 13 | 5 | 1 |
| 70 to 79 | 11 | 4 | 2 |
| 60 to 69 | 10 | 4 | 0 |
| Dissolve CETYLEV Tablets in 150 mL of Water | |||
| 50 to 59 | 8 | 3 | 1 |
| 40 to 49 | 7 | 2 | 4 |
| 30 to 39 | 6 | 2 | 2 |
| 20 to 29 | 4 | 1 | 3 |
|
|||
| Dissolve CETYLEV Tablets in 300 mL of Water | |||
| Body weight (Kg) | Actual Acetylcysteine Dose to be Administered (grams) | Number of CETYLEV Tablets to Dissolve in Water | |
| 2.5 gram tablets | 500 mg tablets | ||
| 100 or greater* | 7.5 | 3 | 0 |
| 90 to 99 | 7 | 2 | 4 |
| 80 to 89 | 6.5 | 2 | 3 |
| 70 to 79 | 5.5 | 2 | 1 |
| 60 to 69 | 5 | 2 | 0 |
| Dissolve CETYLEV Tablets in 150 mL of Water | |||
| 50 to 59 | 4 | 1 | 3 |
| 40 to 49 | 3.5 | 1 | 2 |
| 30 to 39 | 3 | 1 | 1 |
| 20 to 29 | 2 | 0 | 4 |
Patients Weighing 1 to 19 kg
Dissolve two 2.5 gram CETYLEV effervescent tablets in 100 mL of water to create a 50 mg/mL solution. Calculate the loading and maintenance doses using the patient's kilogram weight:
2.4 Recommendations for Repeated Supratherapeutic Acetaminophen Ingestion
Repeated supratherapeutic acetaminophen ingestion (RSI) is an ingestion of acetaminophen at dosages higher than those recommended for extended periods of time. The risk of hepatotoxicity and the recommendations for treatment of acute acetaminophen ingestion (i.e., the Rumack-Matthew nomogram) do not apply to patients with RSI. Therefore, obtain the following information to guide CETYLEV treatment for RSI.
- Acetaminophen serum or plasma concentrations. A reported history of the quantity of acetaminophen ingested is often inaccurate and is not a reliable guide to therapy.
- Laboratory tests to monitor hepatic and renal function and electrolyte and fluid balance: AST, ALT, bilirubin, INR, creatinine, BUN, blood glucose, and electrolytes.
For specific CETYLEV dosage and administration information in patients with RSI, consider contacting your regional poison center at 1-800-222-1222, or alternatively, a special health professional assistance line for acetaminophen overdose at 1-800-525-6115.
3 DOSAGE FORMS AND STRENGTHS
CETYLEV effervescent tablets are supplied as white, round, flat tablets with a lemon mint flavor in the following dosage strengths:
- 500 mg tablets debossed with "I" on one side.
- 2.5 gram tablets debossed with "O" on one side.
CETYLEV tablets contain the inactive ingredient sodium bicarbonate which may be clinically relevant in some patients [see Use in Specific Populations (8.6), Description (11)].
5 WARNINGS AND PRECAUTIONS
5.1 Hypersensitivity Reactions
Hypersensitivity reactions, including generalized urticaria have been observed in patients receiving oral acetylcysteine for acetaminophen overdose. If hypersensitivity reactions occur, CETYLEV should be discontinued unless it is deemed essential for patient management and the reactions can be otherwise controlled.
5.2 Risk of Upper Gastrointestinal Hemorrhage
Occasionally severe and persistent vomiting occurs as a symptom of acute acetaminophen overdose. Treatment with CETYLEV may aggravate the vomiting and increase the risk of upper gastrointestinal hemorrhage in at risk patients (e.g., those with esophageal varices, peptic ulcers, etc.). Consider the risk/benefit for patients at risk of hemorrhage versus the risk of developing hepatic toxicity, and treat with CETYLEV as needed.
6 ADVERSE REACTIONS
The following adverse reactions are described, or described in greater detail, in other sections of the labeling:
- Hypersensitivity Reactions [see Warnings and Precautions (5.1)]
- Risk for Upper Gastrointestinal Hemorrhage [see Warnings and Precautions (5.2)]
The most common adverse reactions have been identified from clinical studies or postmarketing reports of acetylcysteine. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
The most common adverse reactions were nausea, vomiting, other gastrointestinal symptoms, and rash with or without fever.
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Limited published case reports and case series on acetylcysteine use during pregnancy are insufficient to inform a drug-associated risk of birth defects and miscarriage. However, there are clinical considerations [see Clinical Considerations]. In animal reproduction studies, no teratogenic effects were observed with oral administration of acetylcysteine to pregnant rats and rabbits during organogenesis at doses up to 0.6 times the maximum recommended human dose (based on body surface area) of about 560 mg/kg (total dose on first day of treatment) [see Data].
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
Data
Animal Data
No teratogenic effects were observed in embryo-fetal development studies in rats at oral doses up to 2000 mg/kg/day (0.6 times the maximum recommended human dose based on body surface area) or in rabbits at oral doses up to 1000 mg/kg/day (0.6 times the maximum recommended human dose based on body surface area) administered during organogenesis.
8.2 Lactation
Risk Summary
There is no information regarding the presence of acetylcysteine in human milk, or the effects of acetylcysteine on the breastfed infant or on milk production. The development and health benefits of breastfeeding should be considered along with the mother's clinical need for CETYLEV and any potential adverse effects on the breastfed infant from CETYLEV or from the underlying maternal condition.
8.4 Pediatric Use
Pediatric approval, including dosing, is not based on adequate and well-controlled clinical studies. Pediatric dosing recommendations are based on clinical experience [see Dosage and Administration (2.3)].
8.5 Geriatric Use
Clinical studies of acetylcysteine did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience with acetylcysteine has not identified differences in the responses between elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function and of concomitant disease or other drug therapy.
8.6 Patients Sensitive to High Sodium Intake
CETYLEV tablets contain sodium. Consider the total sodium content from dietary and non-dietary sources in patients who may be sensitive to excess sodium intake, such as those with congestive heart failure, hypertension, or renal impairment.
At the recommended dosage an average sized adult (60 kg) may receive a total of 7 grams of sodium (304.3 mEq) on the first day of treatment, 5.3 grams of sodium (230.4 mEq) on the second day of treatment, and 4.4 grams of sodium (191.3 mEq) on the third day of treatment.
If sodium intake is a concern, please refer to Table 3 for the amount of sodium in each tablet [see Description (11)] and to Tables 1 and 2 for the recommended dosage in adults and pediatrics based on body weight in order to calculate the amount of sodium administered to an individual patient [see Dosage and Administration (2.3)].
11 DESCRIPTION
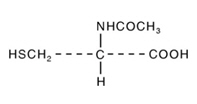
Acetylcysteine is an antidote for the treatment of acetaminophen overdose. It is the N-acetyl derivative of the naturally-occurring amino acid, cysteine. Acetylcysteine is a white crystalline powder that is freely soluble in water, alcohol, practically insoluble in chloroform and in ether with the molecular formula C5H9NO3S, a molecular weight of 163.2, and chemical name of N-acetyl-L-cysteine. Acetylcysteine has the following structural formula:
CETYLEV (acetylcysteine) effervescent tablets for oral solution contain 500 mg or 2.5 grams of acetylcysteine. The following are inactive ingredients: sodium bicarbonate, maltodextrin, lemon flavor, sucralose, peppermint flavor, and edetate disodium.
The amount of sodium in each tablet of CETYLEV is shown in Table 3.
| Tablet Strength | Sodium Bicarbonate (mg)* | Sodium (mg) | Sodium (mEq) |
|---|---|---|---|
|
|||
| 500 mg | 320 mg | 88 mg | 3.8 mEq |
| 2.5 grams | 1600 mg | 438 mg | 19 mEq |
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Acetylcysteine has been shown to reduce the extent of liver injury following acetaminophen overdose. Acetaminophen doses of 150 mg/kg or greater have been associated with hepatotoxicity. Acetylcysteine probably protects the liver by maintaining or restoring the glutathione levels, or by acting as an alternate substrate for conjugation with, and thus detoxification of, the reactive metabolite of acetaminophen.
12.3 Pharmacokinetics
Absorption
After administration of a single oral dose of 11 grams of CETYLEV (dissolved in 300 mL of water) to 29 healthy adult subjects, the mean Cmax (CV%) was 26.5 (29) mcg/mL and mean (CV) AUCinf was 186 (29) hr∙mcg/mL. The median (range) time to reach Cmax (Tmax) was 2 (1 to 3.5) hours.
Distribution
The steady-state volume of distribution (Vd) following administration of an intravenous dose of acetylcysteine was 0.47 liter/kg. The protein binding for acetylcysteine ranges from 66% to 87 %.
Elimination
Metabolism
Acetylcysteine (i.e., N-acetylcysteine) undergoes extensive first pass metabolism and is postulated to form cysteine and disulfides (N,N-diacetylcysteine and N-acetylcysteine). Cysteine is further metabolized to form glutathione and other metabolites.
Excretion
After a single oral dose of [35S]-acetylcysteine 100 mg, between 13 to 38% of the total radioactivity administered was recovered in urine within 24 hours. In a separate study, renal clearance was estimated to be approximately 30% of total body clearance.
In healthy subjects given a single oral dose of 11 grams of CETYLEV, the mean (CV%) terminal plasma half-life (T1/2) was 18.1 (22%) hours.
Specific Populations
Hepatic Impairment
Following a 600 mg intravenous dose of acetylcysteine to subjects with mild (Child Pugh Class A, n=1), moderate (Child-Pugh Class B, n=4) or severe (Child-Pugh Class C; n=4) hepatic impairment and 6 healthy matched controls, mean T1/2 increased by 80%. Also, the mean CL decreased by 30% and the systemic acetylcysteine exposure (mean AUC) increased 1.6-fold in subjects with hepatic impairment compared to subjects with normal hepatic function. These changes are not considered to be clinically meaningful.
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
Carcinogenicity studies in laboratory animals have not been performed with acetylcysteine.
Impairment of Fertility
In a fertility study of acetylcysteine in rats, intravenous administration of 1000 mg/kg/day (0.3 times the recommended human oral dose based on body surface area) caused a profound reduction of fertility in females, which was correlated with morphological changes in oocytes and severe impairment of implantation (18 of 20 mated females had no implantations). The reversibility of this effect was not evaluated. No effects on fertility were observed in female rats at intravenous doses up to 300 mg/kg/day (0.1 times the recommended human oral dose based on body surface area), or in male rats at intravenous doses up to 1000 mg/kg/day. Mating was unaffected in this study.
In a reproduction study of acetylcysteine, male rats were treated orally for 15 weeks prior to mating and during the mating period. A slight non-dose related reduction in fertility was observed at oral doses of 500 and 1000 mg/kg/day (0.1 and 0.3 times the recommended human dose, respectively, based on body surface area).
16 HOW SUPPLIED/STORAGE AND HANDLING
CETYLEV effervescent tablets are supplied as white, round, flat tablets with a lemon mint smell packaged in 2-count peelable foil blister packs in the following dosage strengths:
- 500 mg tablets debossed with "I" on one side; Each carton containing 2-count blister packs (24338-700-02)
- NDC 24338-700-10: 10 pack carton containing 20 tablets
- 2.5 gram tablets debossed with "O" on one side; Each carton containing 2-count blister packs (24338-725-02)
- NDC 24338-725-10: 10 pack carton containing 20 tablets
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information).
Hypersensitivity Reactions
Advise patients that hypersensitivity reactions, including generalized urticaria may occur and to report any signs or symptoms to their healthcare provider immediately [see Warnings and Precautions (5.1)].
Manufactured for:
arbor
PHARMACEUTICALS, LLC
Atlanta, GA 30328
Made in Switzerland by Alpex Pharma SA.
CET-PI-02
Rev. 04/2017
| Patient Information CETYLEV® (SEE-tuh-lev) (acetylcysteine) effervescent tablets for oral solution |
|
| What is CETYLEV?
CETYLEV is a prescription medicine used to prevent or lessen liver damage in people who have taken too much acetaminophen (overdose). |
|
Before taking CETYLEV, tell your healthcare provider about all of your medical conditions, including if you:
|
|
How should I take CETYLEV?
|
|
| What are the possible side effects of CETYLEV? CETYLEV may cause serious side effects, including:
These are not all of the possible side effects of CETYLEV. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088. |
|
How should I store CETYLEV?
|
|
| General information about the safe and effective use of CETYLEV.
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use CETYLEV for a condition for which it was not prescribed. Do not give CETYLEV to other people, even if they have the same symptoms you have. It may harm them. You can ask your pharmacist or healthcare provider for more information about CETYLEV that is written for health professionals. |
|
| What are the ingredients in CETYLEV? Active ingredient: acetylcysteine Inactive ingredients: sodium bicarbonate, maltodextrin, lemon flavor, sucralose, peppermint flavor, and edetate disodium Manufactured for: arbor PHARMACEUTICALS, LLC Atlanta, GA 30328 Made in Switzerland For more information, call 1-866-516-4950 |
|
| This Patient Information has been approved by the U.S. Food and Drug Administration | Issued: January 2016 |
PRINCIPAL DISPLAY PANEL - 500 mg Tablet Blister Pack Carton
NDC 24338-700-10
Cetylev™
(acetylcysteine) effervescent tablets
for oral solution
500 mg
LEMON MINT FLAVOR
Contains 20 tablets (ten 2-count blister packs)
Rx Only

| CETYLEV
acetylcysteine tablet, effervescent |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| CETYLEV
acetylcysteine tablet, effervescent |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| CETYLEV
acetylcysteine tablet, effervescent |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Arbor Pharmaceuticals (781796417) |