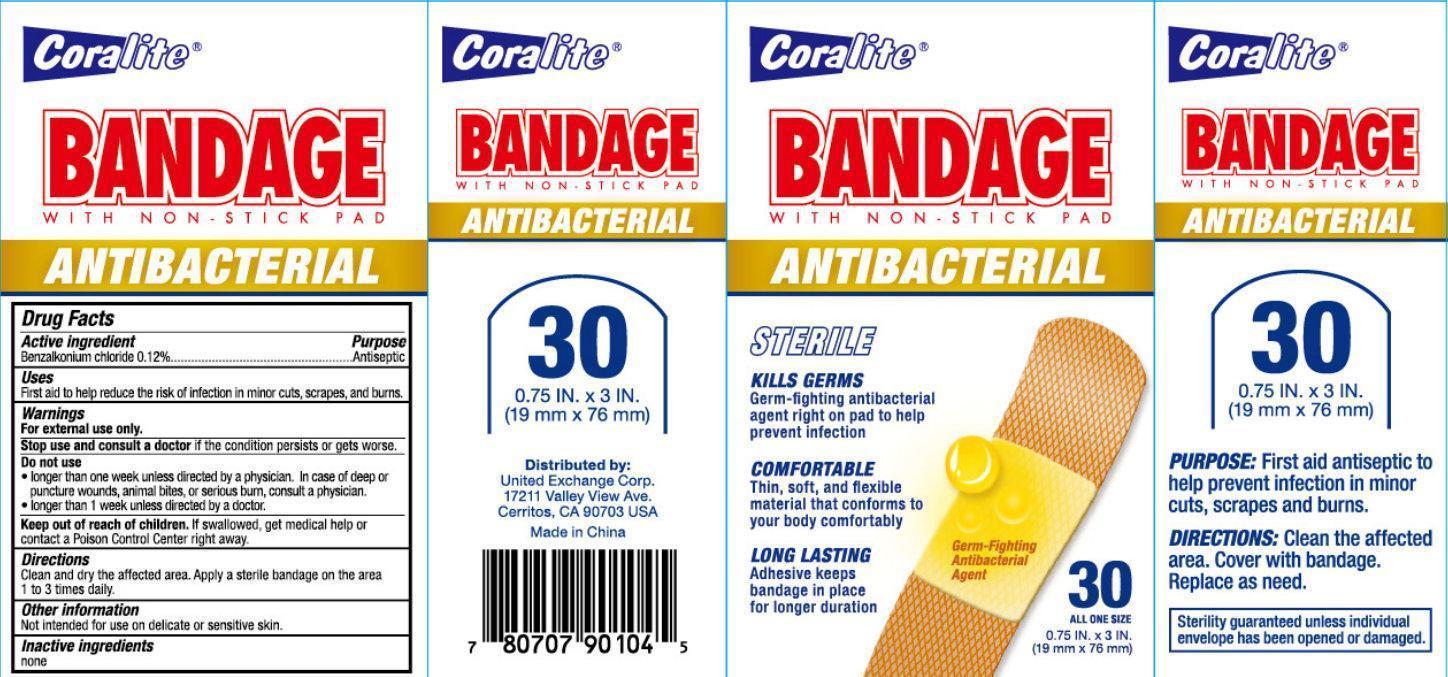
CORALITE ANTIBACTERIAL BANDAGE- benzalkonium chloride patch
UNITED EXCHANGE CORP
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Coralite ANTIBACTERIAL BANDAGE WITH NON STICK PAD
Warnings
For external use only.
| CORALITE ANTIBACTERIAL BANDAGE
benzalkonium chloride patch |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - UNITED EXCHANGE CORP (840130579) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Planet (Suzhou) Medical Products Co., Ltd. | 528194366 | manufacture(65923-001) | |
Revised: 9/2019
Document Id: 93628989-adbd-97a5-e053-2995a90ab7e4
Set id: 7736c2c9-8594-4669-98c8-0f028f455d2a
Version: 100
Effective Time: 20190925
UNITED EXCHANGE CORP