CEDAX- ceftibuten dihydrate capsule
CEDAX- ceftibuten dihydrate suspension
Pernix Therapeutics, LLC
----------
CEDAX®
(ceftibuten capsules)
and
(ceftibuten for oral suspension)
DESCRIPTION
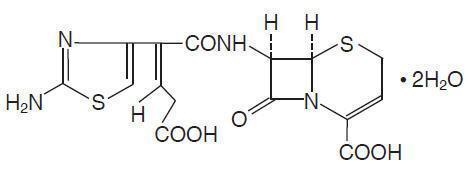
CEDAX (ceftibuten capsules) and (ceftibuten for oral suspension) contain the active ingredient ceftibuten as ceftibuten dihydrate. Ceftibuten dihydrate is a semisynthetic cephalosporin antibiotic for oral administration. Chemically, it is (+)-(6R,7R)-7-[(Z)-2-(2-Amino-4-thiazolyl)-4-carboxycrotonamido]-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid, dihydrate. Its molecular formula is C15H14N4O6S2•2H2O. Its molecular weight is 446.43 as the dihydrate.
Ceftibuten dihydrate has the following structural formula:
CEDAX Capsules contain ceftibuten dihydrate equivalent to 400 mg of ceftibuten. Inactive ingredients contained in the capsule formulation include: magnesium stearate, microcrystalline cellulose, and sodium starch glycolate. The capsule shell and/or band contains gelatin, sodium lauryl sulfate, titanium dioxide, and polysorbate 80. The capsule shell may also contain benzyl alcohol, sodium propionate, edetate calcium disodium, butylparaben, propylparaben, and methylparaben.
CEDAX Oral Suspension after reconstitution contains ceftibuten dihydrate equivalent to 180 mg of ceftibuten per 5 mL. CEDAX Oral Suspension is cherry flavored and contains the inactive ingredients: cherry flavoring, polysorbate 80, silicon dioxide, simethicone, sodium benzoate, sucrose (approximately 1 g/5 mL), titanium dioxide, and xanthan gum.
CLINICAL PHARMACOLOGY
PHARMACOKINETICS
Absorption
CEDAX CAPSULES
Ceftibuten is rapidly absorbed after oral administration of CEDAX Capsules. The plasma concentrations and pharmacokinetic parameters of ceftibuten after a single 400-mg dose of CEDAX Capsules to 12 healthy adult male volunteers (20 to 39 years of age) are displayed in the table below. When CEDAX Capsules were administered once daily for 7 days, the average Cmax was 17.9 µg/mL on day 7. Therefore, ceftibuten accumulation in plasma is about 20% at steady state.
CEDAX ORAL SUSPENSION
Ceftibuten is rapidly absorbed after oral administration of CEDAX Oral Suspension. The plasma concentrations and pharmacokinetic parameters of ceftibuten after a single 9-mg/kg dose of CEDAX Oral Suspension to 32 fasting pediatric patients (6 months to 12 years of age) are displayed in the following table:
| Parameter | Average Plasma Concentration (in µg/mL of ceftibuten after a single 400-mg dose) and Derived Pharmacokinetic Parameters (± 1 SD) (n = 12 healthy adult males) | Average Plasma Concentration (in µg/mL of ceftibuten after a single 9-mg/kg dose) and Derived Pharmacokinetic Parameters (± 1 SD) (n = 32 pediatric patients) |
|---|---|---|
| 1.0 h | 6.1 (5.1) | 9.3 (6.3) |
| 1.5 h | 9.9 (5.9) | 8.6 (4.4) |
| 2.0 h | 11.3 (5.2) | 11.2 (4.6) |
| 3.0 h | 13.3 (3.0) | 9.0 (3.4) |
| 4.0 h | 11.2 (2.9) | 6.6 (3.1) |
| 6.0 h | 5.8 (1.6) | 3.8 (2.5) |
| 8.0 h | 3.2 (1.0) | 1.6 (1.3) |
| 12.0 h | 1.1 (0.4) | 0.5 (0.4) |
| Cmax, µg/mL | 15.0 (3.3) | 13.4 (4.9) |
| Tmax, h | 2.6 (0.9) | 2.0 (1.0) |
| AUC, µg∙h/mL | 73.7 (16.0) | 56.0 (16.9) |
| T½, h | 2.4 (0.2) | 2.0 (0.6) |
| Total body clearance (CI/F) mL/min/kg | 1.3 (0.3) | 2.9 (0.7) |
The absolute bioavailability of CEDAX Oral Suspension has not been determined. The plasma concentrations of ceftibuten in pediatric patients are dose proportional following single doses of CEDAX Capsules of 200 mg and 400 mg and of CEDAX Oral Suspension between 4.5 mg/kg and 9 mg/kg.
Distribution
Protein Binding
Ceftibuten is 65% bound to plasma proteins. The protein binding is independent of plasma ceftibuten concentration.
Tissue Penetration
Bronchial secretions
In a study of 15 adults administered a single 400-mg dose of ceftibuten and scheduled to undergo bronchoscopy, the mean concentrations in epithelial lining fluid and bronchial mucosa were 15% and 37%, respectively, of the plasma concentrations.
Sputum
Ceftibuten sputum levels average approximately 7% of the concomitant plasma ceftibuten level. In a study of 24 adults administered ceftibuten 200 mg bid or 400 mg qd, the average Cmax in sputum (1.5 µg/mL) occurred at 2 hours postdose and the average Cmax in plasma (17 µg/mL) occurred at 2 hours postdose.
Middle-ear fluid (MEF)
In a study of 12 pediatric patients administered 9 mg/kg, ceftibuten MEF area under the curve (AUC) averaged approximately 70% of the plasma AUC. In the same study, Cmax values were 14.3 ± 2.7 µg/mL in MEF at 4 hours postdose and 14.5 ± 3.7 µg/mL in plasma at 2 hours postdose.
Metabolism and Excretion
A study with radiolabeled ceftibuten administered to 6 healthy adult male volunteers demonstrated that cis-ceftibuten is the predominant component in both plasma and urine. About 10% of ceftibuten is converted to the trans-isomer. The trans-isomer is approximately ⅛ as antimicrobially potent as the cis-isomer.
Ceftibuten is excreted in the urine; 95% of the administered radioactivity was recovered either in urine or feces. In 6 healthy adult male volunteers, approximately 56% of the administered dose of ceftibuten was recovered from urine and 39% from the feces within 24 hours. Because renal excretion is a significant pathway of elimination, patients with renal dysfunction and patients undergoing hemodialysis require dosage adjustment (see DOSAGE AND ADMINISTRATION).
Food Effect on Absorption
Food affects the bioavailability of ceftibuten from CEDAX Capsules and CEDAX Oral Suspension.
The effect of food on the bioavailability of CEDAX Capsules was evaluated in 26 healthy adult male volunteers who ingested 400 mg of CEDAX Capsules after an overnight fast or immediately after a standardized breakfast. Results showed that food delays the time of Cmax by 1.75 hours, decreases the Cmax by 18%, and decreases the extent of absorption (AUC) by 8%.
The effect of food on the bioavailability of CEDAX Oral Suspension was evaluated in 18 healthy adult male volunteers who ingested 400 mg of CEDAX Oral Suspension after an overnight fast or immediately after a standardized breakfast. Results obtained demonstrated a decrease in Cmax of 26% and an AUC of 17% when CEDAX Oral Suspension was administered with a high-fat breakfast, and a decrease in Cmax of 17% and an AUC of 12% when CEDAX Oral Suspension was administered with a low-calorie nonfat breakfast (see PRECAUTIONS).
Bioequivalence of Dosage Formulations
A study in 18 healthy adult male volunteers demonstrated that a 400-mg dose of CEDAX Capsules produced equivalent concentrations to a 400-mg dose of CEDAX Oral Suspension. Average Cmax values were 15.6 (3.1) µg/mL for the capsule and 17.0 (3.2) µg/mL for the suspension. Average AUC values were 80.1 (14.4) µg∙hr/mL for the capsule and 87.0 (12.2) µg∙hr/mL for the suspension.
Special Populations
Geriatric patients
Ceftibuten pharmacokinetics have been investigated in elderly (65 years of age and older) men (n = 8) and women (n = 4). Each volunteer received ceftibuten 200-mg capsules twice daily for 3½ days. The average Cmax was 17.5 (3.7) µg/mL after 3½ days of dosing compared to 12.9 (2.1) µg/mL after the first dose; ceftibuten accumulation in plasma was 40% at steady state. Information regarding the renal function of these volunteers was not available; therefore, the significance of this finding for clinical use of CEDAX Capsules in elderly patients is not clear. Ceftibuten dosage adjustment in elderly patients may be necessary (see DOSAGE AND ADMINISTRATION).
Patients with renal insufficiency
Ceftibuten pharmacokinetics have been investigated in adult patients with renal dysfunction. The ceftibuten plasma half-life increased and apparent total clearance (CI/F) decreased proportionately with increasing degree of renal dysfunction. In 6 patients with moderate renal dysfunction (creatinine clearance 30 to 49 mL/min), the plasma half-life of ceftibuten increased to 7.1 hours and CI/F decreased to 30 mL/min. In 6 patients with severe renal dysfunction (creatinine clearance 5 to 29 mL/min), the half-life increased to 13.4 hours and CI/F decreased to 16 mL/min. In 6 functionally anephric patients (creatinine clearance <5 mL/min), the half-life increased to 22.3 hours and CI/F decreased to 11 mL/min (a 7- to 8-fold change compared to healthy volunteers). Hemodialysis removed 65% of the drug from the blood in 2 to 4 hours. These changes serve as the basis for dosage adjustment recommendations in adult patients with mild to severe renal dysfunction (see DOSAGE AND ADMINISTRATION).
Microbiology
Ceftibuten exerts its bactericidal action by binding to essential target proteins of the bacterial cell wall. This binding leads to inhibition of cell-wall synthesis.
Ceftibuten is stable in the presence of most plasmid-mediated beta-lactamases, but it is not stable in the presence of chromosomally-mediated cephalosporinases produced in organisms such as Bacteroides, Citrobacter, Enterobacter, Morganella, and Serratia. Like other beta-lactam agents, ceftibuten should not be used against strains resistant to beta-lactams due to general mechanisms such as permeability or penicillin-binding protein changes like penicillin-resistant S. pneumoniae.
Ceftibuten has been shown to be active against most strains of the following organisms both in vitro and in clinical infections (see INDICATIONS AND USAGE):
Gram-positive aerobes:
- Streptococcus pneumoniae (penicillin-susceptible strains only)
- Streptococcus pyogenes
Gram-negative aerobes:
- Haemophilus influenzae (including β-lactamase-producing strains)
- Moraxella catarrhalis (including β-lactamase-producing strains)
There are no known organisms which are potential pathogens in the indications approved for ceftibuten for which ceftibuten exhibits in vitro activity but for which the safety and efficacy of ceftibuten in treating clinical infections due to these organisms, have not been established in adequate and well-controlled trials.
NOTE: Ceftibuten is INACTIVE in vitro against Acinetobacter, Bordetella, Campylobacter, Enterobacter, Enterococcus, Flavobacterium, Hafnia, Listeria, Pseudomonas, Staphylococcus, and Streptococcus (except pneumoniae and pyogenes) species. In addition, it shows little in vitro activity against most anaerobes, including most species of Bacteroides.
Susceptibility testing
Dilution Techniques
Quantitative methods are used to determine antimicrobial minimal inhibitory concentrations (MICs). These MICs provide estimates of the susceptibility of bacteria to antimicrobial compounds. The MICs should be determined using a standardized procedure. Standardized procedures are based on a dilution method (broth, agar, or microdilution) or equivalent with standardized inoculum concentrations and standardized concentrations of ceftibuten powder. The MIC values should be interpreted according to the following criteria when testing Haemophilus species using Haemophilus Test Media (HTM):
| MIC (µg/mL) | Interpretation | |||
| ≤2 | (S) Susceptible |
The current absence of resistant strains precludes defining any categories other than "Susceptible". Strains yielding results suggestive of a "Nonsusceptible" category should be submitted to a reference laboratory for further testing.
A report of "Susceptible" implies that an infection due to the strain may be appropriately treated with the dosage of antimicrobial agent recommended for that type of infection and infecting species, unless otherwise contraindicated.
Ceftibuten is indicated for penicillin-susceptible only strains of Streptococcus pneumoniae. A pneumococcal isolate that is susceptible to penicillin (MIC ≤0.06 µg/mL) can be considered susceptible to ceftibuten for approved indications. Testing of ceftibuten against penicillin-intermediate or penicillin-resistant isolates is not recommended. Reliable interpretive criteria for ceftibuten are not currently available. Physicians should be informed that clinical response rates with ceftibuten may be lower in strains that are not penicillin-susceptible.
Standardized susceptibility test procedures require the use of laboratory control microorganisms to control the technical aspect of laboratory procedures. Standard ceftibuten powder should provide the following MIC values:
| Organism | MIC range (µg/mL) | ||||
| Haemophilus influenzae ATCC 49247 | 0.25-1.0 | ||||
Diffusion Techniques
Quantitative methods that require measurement of zone diameters also provide estimates of the susceptibility of bacteria to antimicrobial compounds. One such standardized procedure requires the use of standardized inoculum concentrations. This procedure uses paper disks impregnated with 30 µg of ceftibuten to test the susceptibility of microorganisms to ceftibuten.
Reports from the laboratory providing results of the standard single-disk susceptibility test with a 30-µg ceftibuten disk should be interpreted according to the following criteria when testing Haemophilus species using Haemophilus Test Media (HTM):
| Zone diameter (mm) | Interpretation | |||
| ≥28 | (S) Susceptible |
The current absence of resistant strains precludes defining any categories other than "Susceptible". Strains yielding results suggestive of a "Nonsusceptible" category should be submitted to a reference laboratory for further testing.
Interpretation should be as stated above for results using dilution techniques.
Ceftibuten is indicated for penicillin-susceptible only strains of Streptococcus pneumoniae. Pneumococcal isolates with oxacillin zone sizes of ≥20 mm are susceptible to penicillin and can be considered susceptible for approved indications. Reliable disk diffusion tests for ceftibuten do not yet exist.
As with standardized dilution techniques, diffusion methods require the use of laboratory control microorganisms that are used to control the technical aspects of the laboratory procedures. For the diffusion technique, the 30-µg ceftibuten disk should provide the following zone diameters in these laboratory test quality control strains:
| Organism | Zone diameter (mm) | ||||
| Haemophilus influenzae ATCC 49247 | 28-34 | ||||
Cephalosporin-class disks should not be used to test for susceptibility to ceftibuten.
INDICATIONS AND USAGE
CEDAX (ceftibuten) is indicated for the treatment of individuals with mild-to-moderate infections caused by susceptible strains of the designated microorganisms in the specific conditions listed below (see DOSAGE AND ADMINISTRATION and CLINICAL STUDIES sections).
Acute Bacterial Exacerbations of Chronic Bronchitis due to Haemophilus influenzae (including β-lactamase-producing strains), Moraxella catarrhalis (including β-lactamase-producing strains), or Streptococcus pneumoniae (penicillin-susceptible strains only).
NOTE: In acute bacterial exacerbations of chronic bronchitis clinical trials where Moraxella catarrhalis was isolated from infected sputum at baseline, ceftibuten clinical efficacy was 22% less than control.
Acute Bacterial Otitis Media due to Haemophilus influenzae (including β-lactamase-producing strains), Moraxella catarrhalis (including β-lactamase-producing strains), or Streptococcus pyogenes.
NOTE: Although ceftibuten used empirically was equivalent to comparators in the treatment of clinically and/or microbiologically documented acute otitis media, the efficacy against Streptococcus pneumoniae was 23% less than control. Therefore, ceftibuten should be given empirically only when adequate antimicrobial coverage against Streptococcus pneumoniae has been previously administered.
Pharyngitis and Tonsillitis due to Streptococcus pyogenes.
NOTE: Only penicillin by the intramuscular route of administration has been shown to be effective in the prophylaxis of rheumatic fever. Ceftibuten is generally effective in the eradication of Streptococcus pyogenes from the oropharynx; however, data establishing the efficacy of the CEDAX product for the prophylaxis of subsequent rheumatic fever are not available.
CONTRAINDICATIONS
CEDAX (ceftibuten) is contraindicated in patients with known allergy to the cephalosporin group of antibiotics.
WARNINGS
BEFORE THERAPY WITH THE CEDAX PRODUCT IS INSTITUTED, CAREFUL INQUIRY SHOULD BE MADE TO DETERMINE WHETHER THE PATIENT HAS HAD PREVIOUS HYPERSENSITIVITY REACTIONS TO CEFTIBUTEN, OTHER CEPHALOSPORINS, PENICILLINS, OR OTHER DRUGS. IF THIS PRODUCT IS TO BE GIVEN TO PENICILLIN-SENSITIVE PATIENTS, CAUTION SHOULD BE EXERCISED BECAUSE CROSS HYPERSENSITIVITY AMONG BETA-LACTAM ANTIBIOTICS HAS BEEN CLEARLY DOCUMENTED AND MAY OCCUR IN UP TO 10% OF PATIENTS WITH A HISTORY OF PENICILLIN ALLERGY. IF AN ALLERGIC REACTION TO THE CEDAX PRODUCT OCCURS, DISCONTINUE THE DRUG. SERIOUS ACUTE HYPERSENSITIVITY REACTIONS MAY REQUIRE TREATMENT WITH EPINEPHRINE AND OTHER EMERGENCY MEASURES, INCLUDING OXYGEN, INTRAVENOUS FLUIDS, INTRAVENOUS ANTIHISTAMINES, CORTICOSTEROIDS, PRESSOR AMINES, AND AIRWAY MANAGEMENT, AS CLINICALLY INDICATED.
Pseudomembranous colitis has been reported with nearly all antibacterial agents, including ceftibuten, and may range in severity from mild to life threatening. Therefore, it is important to consider this diagnosis in patients who present with diarrhea subsequent to the administration of antibacterial agents.
Treatment with antibacterial agents alters normal flora of the colon and may permit overgrowth of clostridia. Studies indicate that a toxin produced by Clostridium difficile is one primary cause of "antibiotic-associated colitis".
After the diagnosis of pseudomembranous colitis has been established, appropriate therapeutic measures should be initiated. Mild cases of pseudomembranous colitis usually respond to drug discontinuation alone. In moderate to severe cases, consideration should be given to management with fluids and electrolytes, protein supplementation, and treatment with an antibacterial drug clinically effective against Clostridium difficile.
PRECAUTIONS
General
As with other broad-spectrum antibiotics, prolonged treatment may result in the possible emergence and overgrowth of resistant organisms. Careful observation of the patient is essential. If superinfection occurs during therapy, appropriate measures should be taken.
The dose of ceftibuten may require adjustment in patients with varying degrees of renal insufficiency, particularly in patients with creatinine clearance less than 50 mL/min or undergoing hemodialysis (see DOSAGE AND ADMINISTRATION). Ceftibuten is readily dialyzable. Dialysis patients should be monitored carefully, and administration of ceftibuten should occur immediately following dialysis.
Ceftibuten should be prescribed with caution to individuals with a history of gastrointestinal disease, particularly colitis.
Information to Patients
Patients should be informed that:
- If the patient is diabetic, he/she should be informed that CEDAX Oral Suspension contains 1 gram sucrose per teaspoon of suspension.
- CEDAX Oral Suspension should be taken at least 2 hours before a meal or at least 1 hour after a meal (see CLINICAL PHARMACOLOGY, Food Effect on Absorption).
Drug Interactions
Theophylline
Twelve healthy male volunteers were administered one 200-mg ceftibuten capsule twice daily for 6 days. With the morning dose of ceftibuten on day 6, each volunteer received a single intravenous infusion of theophylline (4 mg/kg). The pharmacokinetics of theophylline were not altered. The effect of ceftibuten on the pharmacokinetics of theophylline administered orally has not been investigated.
Antacids or H2-receptor antagonists
The effect of increased gastric pH on the bioavailability of ceftibuten was evaluated in 18 healthy adult volunteers. Each volunteer was administered one 400-mg ceftibuten capsule. A single dose of liquid antacid did not affect the Cmax or AUC of ceftibuten; however, 150 mg of ranitidine q12h for 3 days increased the ceftibuten Cmax by 23% and ceftibuten AUC by 16%. The clinical relevance of these increases is not known.
Drug/Laboratory Test Interactions
There have been no chemical or laboratory test interactions with ceftibuten noted to date. False-positive direct Coombs' tests have been reported during treatment with other cephalosporins. Therefore, it should be recognized that a positive Coombs' test could be due to the drug. The results of assays using red cells from healthy subjects to determine whether ceftibuten would cause direct Coombs' reactions in vitro showed no positive reaction at ceftibuten concentrations as high as 40 µg/mL.
Carcinogenesis, Mutagenesis, Impairment of Fertility
Long-term animal studies have not been performed to evaluate the carcinogenic potential of ceftibuten. No mutagenic effects were seen in the following studies: in vitro chromosome assay in human lymphocytes, in vivo chromosome assay in mouse bone marrow cells, Chinese Hamster Ovary (CHO) cell point mutation assay at the hypoxanthine-guanine phosphoribosyl transferase (HGPRT) locus, and in a bacterial reversion point mutation test (Ames). No impairment of fertility occurred when rats were administered ceftibuten orally up to 2000 mg/kg/day (approximately 43 times the human dose based on mg/m2/day).
Pregnancy
Teratogenic effects
Pregnancy Category B
Ceftibuten was not teratogenic in the pregnant rat at oral doses up to 400 mg/kg/day (approximately 8.6 times the human dose based on mg/m2/day). Ceftibuten was not teratogenic in the pregnant rabbit at oral doses up to 40 mg/kg/day (approximately 1.5 times the human dose based on mg/m2/day) and has revealed no evidence of harm to the fetus. There are no adequate and well-controlled studies in pregnant women. Because animal reproduction studies are not always predictive of human response, this drug should be used during pregnancy only if clearly needed.
Labor and Delivery
Ceftibuten has not been studied for use during labor and delivery. Its use during such clinical situations should be weighed in terms of potential risk and benefit to both mother and fetus.
Nursing Mothers
It is not known whether ceftibuten (at recommended dosages) is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when ceftibuten is administered to a nursing woman.
ADVERSE EVENTS
Clinical Trials
CEDAX CAPSULES (adult patients)
In clinical trials, 1728 adult patients (1092 US and 636 international) were treated with the recommended dose of ceftibuten capsules (400 mg per day). There were no deaths or permanent disabilities thought due to drug toxicity in any of the patients in these studies. Thirty-six of 1728 (2%) patients discontinued medication due to adverse events thought by the investigators to be possibly, probably, or almost certainly related to drug toxicity. The discontinuations were primarily for gastrointestinal disturbances, usually diarrhea, vomiting, or nausea. Six of 1728 (0.3%) patients were discontinued due to rash or pruritus thought related to ceftibuten administration.
In the US trials, the following adverse events were thought by the investigators to be possibly, probably, or almost certainly related to ceftibuten capsules in multipledose clinical trials (n = 1092 ceftibuten-treated patients).
| ADVERSE REACTIONS CEFTIBUTEN CAPSULES US CLINICAL TRIALS IN ADULT PATIENTS (n = 1092) |
||
|---|---|---|
| Incidence equal to or greater than 1% | Nausea | 4% |
| Headache | 3% | |
| Diarrhea | 3% | |
| Dyspepsia | 2% | |
| Dizziness | 1% | |
| Abdominal pain | 1% | |
| Vomiting | 1% | |
| Incidence less than 1% but greater than 0.1% | Anorexia, Constipation, Dry mouth, Dyspnea, Dysuria, Eructation, Fatigue, Flatulence, Loose stools, Moniliasis, Nasal congestion, Paresthesia, Pruritus, Rash, Somnolence, Taste perversion, Urticaria, Vaginitis | |
| LABORATORY VALUE CHANGES*
CEFTIBUTEN CAPSULES US CLINICAL TRIALS IN ADULT PATIENTS |
||
|---|---|---|
|
||
| Incidence equal to or greater than 1% | ↑ BUN | 4% |
| ↑ Eosinophils | 3% | |
| ↓ Hemoglobin | 2% | |
| ↑ ALT (SGPT) | 1% | |
| ↑ Bilirubin | 1% | |
| Incidence less than 1% but greater than 0.1% | ↑ Alk phosphatase | |
| ↑ Creatinine | ||
| ↑ Platelets | ||
| ↓ Platelets | ||
| ↓ Leukocytes | ||
| ↑ AST (SGOT) | ||
CEDAX ORAL SUSPENSION (pediatric patients)
In clinical trials, 1152 pediatric patients (772 US and 380 international), 97% of whom were younger than 12 years of age, were treated with the recommended dose of ceftibuten (9 mg/kg once daily up to a maximum dose of 400 mg per day) for 10 days. There were no deaths, life-threatening adverse events, or permanent disabilities in any of the patients in these studies. Eight of 1152 (<1%) patients discontinued medication due to adverse events thought by the investigators to be possibly, probably, or almost certainly related to drug toxicity. The discontinuations were primarily (7 out of 8) for gastrointestinal disturbances, usually diarrhea or vomiting. One patient was discontinued due to a cutaneous rash thought possibly related to ceftibuten administration.
In the US trials, the following adverse events were thought by the investigators to be possibly, probably, or almost certainly related to ceftibuten oral suspension in multipledose clinical trials (n = 772 ceftibuten-treated patients).
| ADVERSE REACTIONS CEFTIBUTEN ORAL SUSPENSION US CLINICAL TRIALS IN PEDIATRIC PATIENTS (n = 772) |
||
|---|---|---|
|
||
| Incidence equal to or greater than 1% | Diarrhea* | 4% |
| Vomiting | 2% | |
| Abdominal pain | 2% | |
| Loose stools | 2% | |
| Incidence less than 1% but greater than 0.1% | Agitation, Anorexia, Dehydration, Diaper dermatitis, Dizziness, Dyspepsia, Fever, Headache, Hematuria, Hyperkinesia, Insomnia, Irritability, Nausea, Pruritus, Rash, Rigors, Urticaria | |
| LABORATORY VALUE CHANGES*
CEFTIBUTEN ORAL SUSPENSION US CLINICAL TRIALS IN PEDIATRIC PATIENTS |
||
|---|---|---|
|
||
| Incidence equal to or greater than 1% | ↑ Eosinophils | 3% |
| ↑ BUN | 2% | |
| ↓ Hemoglobin | 1% | |
| ↑ Platelets | 1% | |
| Incidence less than 1% but greater than 0.1% | ↑ ALT (SGPT) | |
| ↑ AST (SGOT) | ||
| ↑ Alk phosphatase | ||
| ↑ Bilirubin | ||
| ↑ Creatinine | ||
In Post-marketing Experience
The following adverse experiences have been reported during worldwide post-marketing surveillance: aphasia, jaundice, melena, psychosis, serum sickness-like reactions, stridor, Stevens-Johnson syndrome, and toxic epidermal necrolysis.
Cephalosporin-class Adverse Reactions
In addition to the adverse reactions listed above that have been observed in patients treated with ceftibuten capsules, the following adverse events and altered laboratory tests have been reported for cephalosporin-class antibiotics:
- allergic reactions, anaphylaxis, drug fever, Stevens-Johnson syndrome, renal dysfunction, toxic nephropathy, hepatic cholestasis, aplastic anemia, hemolytic anemia, hemorrhage, false-positive test for urinary glucose, neutropenia, pancytopenia, and agranulocytosis. Pseudomembranous colitis; onset of symptoms may occur during or after antibiotic treatment (see WARNINGS).
Several cephalosporins have been implicated in triggering seizures, particularly in patients with renal impairment when the dosage was not reduced (see DOSAGE AND ADMINISTRATION and OVERDOSAGE). If seizures associated with drug therapy occur, the drug should be discontinued. Anticonvulsant therapy can be given if clinically indicated.
OVERDOSAGE
Overdosage of cephalosporins can cause cerebral irritation leading to convulsions. Ceftibuten is readily dialyzable and significant quantities (65% of plasma concentrations) can be removed from the circulation by a single hemodialysis session. Information does not exist with regard to removal of ceftibuten by peritoneal dialysis.
DOSAGE AND ADMINISTRATION
The recommended doses of CEDAX Oral Suspension are presented in the table below. CEDAX Oral Suspension must be administered at least 2 hours before or 1 hour after a meal.
| Type of infection (as qualified in the INDICATIONS AND USAGE section of this labeling) | Daily Maximum Dose | Dose and Frequency | Duration |
|---|---|---|---|
| ADULTS (12 years of age and older): | 400 mg | 400 mg QD | 10 days |
| Acute Bacterial Exacerbations of Chronic Bronchitis due to H. influenzae (including β-lactamase-producing strains), M. catarrhalis (including β-lactamase-producing strains), or Streptococcus pneumoniae (penicillin-susceptible strains only). (See INDICATIONS AND USAGE - NOTE.) Pharyngitis and tonsillitis due to S. pyogenes. Acute Bacterial Otitis Media due to H. influenzae (including β-lactamase-producing strains), M. catarrhalis (including β-lactamase-producing strains), or S. pyogenes. (See INDICATIONS AND USAGE - NOTE.) | |||
| PEDIATRIC PATIENTS: | 400 mg | 9 mg/kg QD | 10 days |
| Pharyngitis and tonsillitis due to S. pyogenes. Acute Bacterial Otitis Media due to H. influenzae (including β-lactamase-producing strains), and M. catarrhalis (including β-lactamase-producing strains), or S. pyogenes. (See INDICATIONS AND USAGE - NOTE.) |
| CEFTIBUTEN ORAL SUSPENSION PEDIATRIC DOSAGE CHART |
|
|---|---|
| CHILD'S WEIGHT | 180 mg/5 mL |
| Pediatric patients weighing more than 45 kg should receive the maximum daily dose of 400 mg. | |
| 10 kg 22 lbs | 1/2 tsp QD |
| 20 kg 44 lbs | 1 tsp QD |
| 40 kg 88 lbs | 2 tsp QD |
Renal Impairment
CEDAX Capsules and CEDAX Oral Suspension may be administered at normal doses in the presence of impaired renal function with creatinine clearance of 50 mL/min or greater. The recommendations for dosing in patients with varying degrees of renal insufficiency are presented in the following table.
| Creatinine Clearance | Recommended Dosing Schedules |
|---|---|
| (mL/min) | |
| >50 | 9 mg/kg or 400 mg Q24h |
| (normal dosing schedule) | |
| 30-49 | 4.5 mg/kg or 200 mg Q24h |
| 5-29 | 2.25 mg/kg or 100 mg Q24h |
Hemodialysis Patients
In patients undergoing hemodialysis two or three times weekly, a single 400-mg dose of ceftibuten capsules or a single dose of 9 mg/kg (maximum of 400 mg of ceftibuten) oral suspension may be administered at the end of each hemodialysis session.
Directions for Mixing CEDAX Oral Suspension
| Final Concentration | Bottle Size | Amount of Water | Directions |
|---|---|---|---|
| After mixing, the suspension may be kept for 14 days and must be stored in the refrigerator. Keep tightly closed. Shake well before each use. Discard any unused portion after 14 days. |
|||
| 180 mg per 5 mL | 30 mL | Suspend in 28 mL of water | First tap the bottle to loosen powder. Then add water in two portions, shaking well after each aliquot. |
| 60 mL | Suspend in 53 mL of water | ||
HOW SUPPLIED
CEDAX Capsules, containing 400 mg of ceftibuten (as ceftibuten dihydrate) are white, opaque capsules imprinted with the product name and strength, are available as follows:
- 20 Capsules/Bottle (NDC 65224-800-22)
CEDAX Oral Suspension is an off-white to cream-colored powder that, when reconstituted as directed, contains ceftibuten equivalent to 180 mg/5 mL, supplied as follows:
180 mg/5 mL
- 36 mg/mL 30-mL Bottle (NDC 65224-804-30)
- 36 mg/mL 60-mL Bottle (NDC 65224-804-02)
CLINICAL STUDIES
Acute Bacterial Exacerbations of Chronic Bronchitis
Three clinical trials (two domestic, the third abroad) have been conducted testing ceftibuten in the treatment of acute exacerbations of chronic bronchitis (AECB). Overall, the clinical outcome among patients who had signs and symptoms of AECB, who had a gram stain showing a predominance of PMNs and few epithelial cells, and who were evaluated at approximately 1 to 2 weeks after completing therapy were equivalent to comparators. The bacterial eradication rates of specific pathogens are presented below.
| Ceftibuten 400 mg QD | Control | |
|---|---|---|
| Bacteriological Eradication Rates | ||
| Haemophilus influenzae | 45/62 (73%) | 26/36 (72%) |
| H. parainfluenzae | 10/10 | 4/6 |
| Moraxella catarrhalis | 33/46 (72%) | 32/34 (94%) |
| Streptococcus pneumoniae | 23/35 (66%) | 14/20 (70%) |
Acute Bacterial Otitis Media
Four clinical trials (three domestic, the fourth abroad) have been conducted testing ceftibuten in the treatment of acute bacterial otitis media. Overall, the clinical outcome among patients who had signs and symptoms of acute bacterial otitis media and who were evaluated at approximately 1 to 2 weeks after completing therapy were equivalent to comparators. Tympanocentesis was performed on patients in three of the above-mentioned studies; the bacterial eradication rates of specific pathogens are presented below.
| Ceftibuten 9 mg/kg QD | Control | |
|---|---|---|
| Bacteriological Eradication Rates | ||
| Haemophilus influenzae | 56/67 (81%) | 29/38 (76%) |
| Moraxella catarrhalis | 20/26 (77%) | 13/17 (77%) |
| Streptococcus pneumoniae | 68/105 (65%) | 35/40 (88%) |
| Streptococcus pyogenes | 13/15 (87%) | 5/5 |
REFERENCES
- National Committee for Clinical Laboratory Standards. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically – Third Edition. Approved Standard NCCLS Document M7-A3, Vol. 13, No. 25, NCCLS, Villanova, PA. December, 1993.
- National Committee for Clinical Laboratory Standards. Performance Standards for Antimicrobial Disk Susceptibility Tests – Fifth Edition. Approved Standard NCCLS Document M2-A5, Vol. 13, No. 24, NCCLS, Villanova, PA. December, 1993.
For inquires call 1-800-793-2145
Manufactured by:
Merck Sharp & Dohme Corp., a subsidiary of
MERCK & CO., INC.
Whitehouse Station, NJ 08889, USA
Distributed by Pernix Therapeutics, LLC
Morristown, NJ 07960, USA
Rev. 03/15
34459029
PRINCIPAL DISPLAY PANEL - 400 mg Capsule Bottle Label
NDC 65224-800-22
CEDAX®
(ceftibuten
capsules)
For Oral Administration
Rx only
20 Capsules
PERNIX
THERAPEUTICS

| CEDAX
ceftibuten dihydrate capsule |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| CEDAX
ceftibuten dihydrate suspension |
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
| CEDAX
ceftibuten dihydrate suspension |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Labeler - Pernix Therapeutics, LLC (004672296) |

