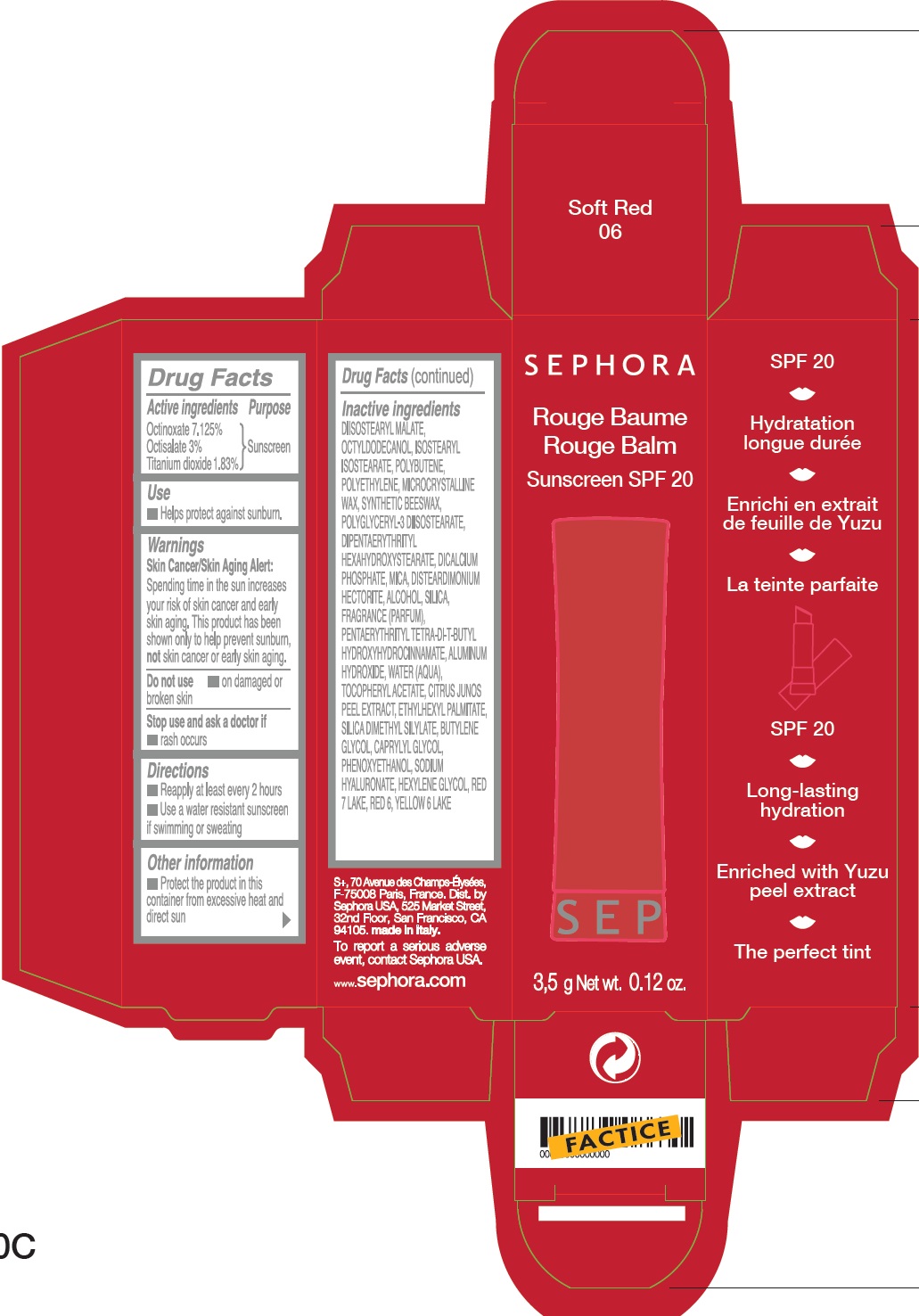
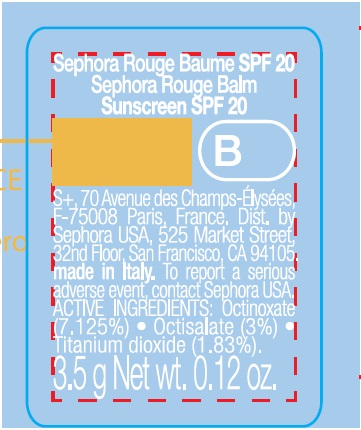
SEPHORA ROUGE BALM SUNSCREEN SPF20 B06-SOFT RED- octinoxate, octisalate, titanium dioxide lipstick
S+
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
SEPHORA ROUGE BALM SUNSCREEN SPF20 B06-Soft Red
Warnings
Sking Cancer/Skin Aging Alert: Spending time in the sun increases your risk of skin cancer and early skin aging. This product has been shown only to help prevent sunburn, not skin cancer or early skin aging.
Inactive ingredients
DIISOSTEARYL MALATE, OCTYLDODECANOL, ISOSTEARYL ISOSTEARATE, POLYBUTENE, POLYETHYLENE, MICROCRYSTALLINE WAX,
SYNTHETIC BEESWAX, POLYGLYCERYL-3 DIISOSTEARATE, DIPENTAERYTHRITYL HEXAHYDROXYSTEARATE, DICALCIUM PHOSPHATE, MICA,
DISTEARDIMONIUM HECTORITE, ALCOHOL, SILICA, FRAGRANCE (PARFUM), PENTAERYTHRITYL TETRA-DI-T-BUTYL HYDROXYHYDROCINNAMATE,
ALUMINUM HYDROXIDE, WATER (AQUA), TOCOPHEROL ACETATE, CITRUS JUNOS PEEL EXTRACT, ETHYLHEXYL PALMITATE, SILICA DIMETHYL SILYLATE, BUTYLENE GLYCOL, CAPRYLYL GLYCOL, PHENOXYETHANOL, SODIUM HYALURONATE, HEXYLENE GLYCOL, RED 7 LAKE,RED 6, YELLOW 6 LAKE
| SEPHORA ROUGE BALM SUNSCREEN SPF20 B06-SOFT RED
octinoxate, octisalate, titanium dioxide lipstick |
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - S+ (572406531) |