MCKESSON HAND SANITIZER- alcohol gel
McKesson Medical-Surgical
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Warnings:
FLAMMABLE, keep away from fire or flame. For external use only.
- Do not use in the eyes
- Discontinue use if irritation and redness develop. If condition persists for more than 72 hours, consult a doctor.
Other information:
Stor below 110°F. Avoid direct sunlight, fluorescent lighting, heat, and moisture.
Inactive Ingredients:
Water, Aloe Barbadensis Leaf Juice, Glycerin, Propylene Glycol, Carbomer, Fragrance, Aminomethyl Propanol
Principal Display Panel - Case Label
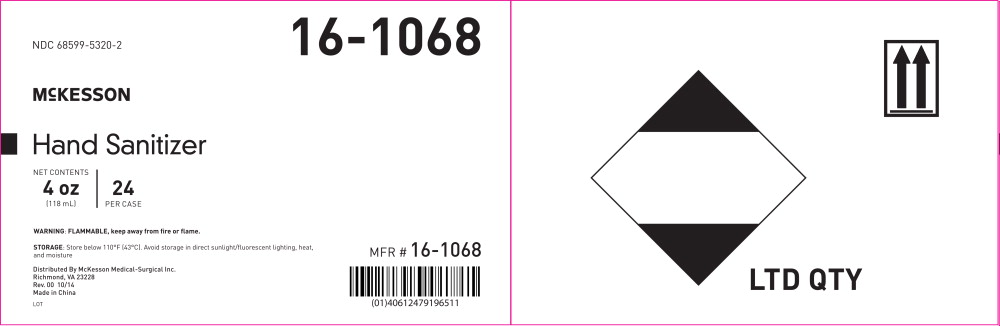
NDC 68599-5320-2
16-1068
MCKESSON
Hand Sanitizer
NET CONTENTS
4 oz
(118 mL)
24
PER CASE
WARNING: FLAMMABLE, keep away from fire or flame.
STORAGE: Store below 110°F (43°C). Avoid storage in direct sunlight/fluorescent lighting, heat, and moisture
Distributed By McKesson Medical-Surgical Inc.
Richmond, VA 23228
Rev. 00 10/14
Made in China
LOT
MFR # 16-1068
| MCKESSON HAND SANITIZER
alcohol gel |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Labeler - McKesson Medical-Surgical (023904428) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Nantong Health and Beyond | 421280161 | manufacture(68599-5320) | |