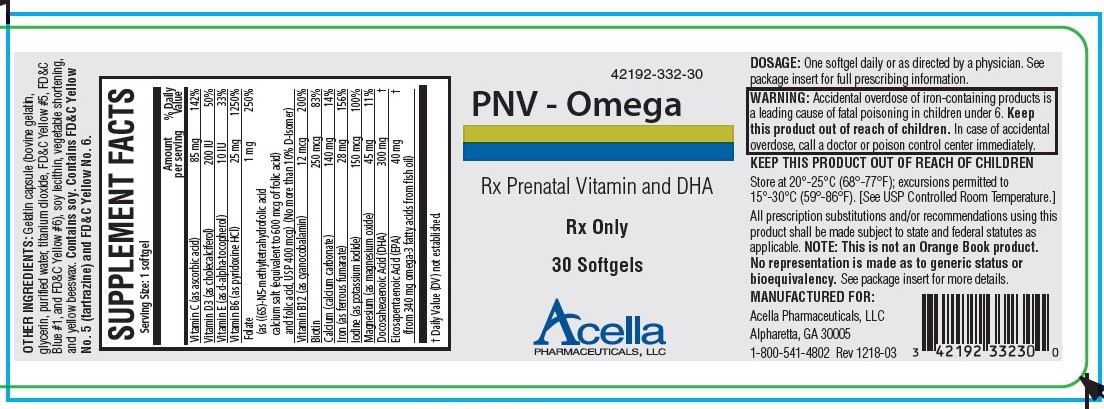
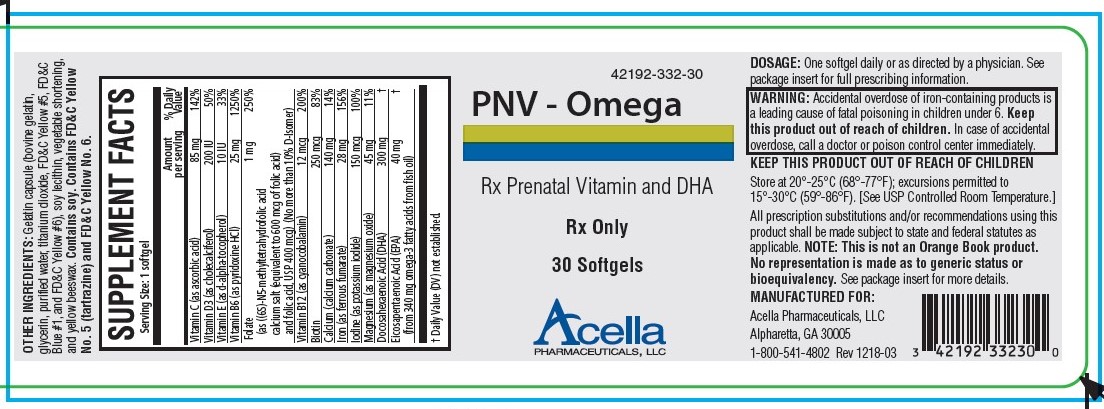
Label: PNV-OMEGA- ascorbic acid, cholecalciferol, .alpha.-tocopherol, pyridoxine, folic acid, cyanocobalamin, calcium corbonate, ferrous fumarate, potassium iodide, magnesium, doconexent, icosapent capsule, gelatin coated
- NDC Code(s): 42192-332-30
- Packager: Acella Pharmaceuticals, LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated January 15, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
-
DESCRIPTION
PNV-Omega is a prescription prenatal/postnatal dietary supplement softgel capsule that contains multivitamins and minerals along with essential fatty acids. Each softgel is green in color, opaque and imprinted with “332” on one side.
OTHER INGREDIENTS
Gelatin capsule (bovine gelatin, glycerin, purified water, titanium dioxide, FD&C Yellow #5, FD&C Blue #1, and FD&C Yellow #6), soy lecithin, vegetable shortening, and yellow beeswax. Contains soy. Contains FD&C Yellow No. 5 (tartrazine) and FD&C Yellow No. 6.
-
WARNING
Ingestion of more than 3 grams of omega-3 fatty acids (such as DHA) per day has been shown to have potential antithrombotic effects, including an increased bleeding time and International Normalized Ratio (INR). Administration of omega-3 fatty acids should be avoided in patients taking anticoagulants and in those known to have an inherited or acquired predisposition to bleeding.
- BOXED WARNING (What is this?)
- PRECAUTIONS
- ADVERSE REACTIONS
- DOSAGE AND ADMINISTRATION
-
HOW SUPPLIED
PNV - Omega is supplied in child-resistant bottles of 30 softgels (42192-332-30). The listed product is not a National Drug Code, but has merely been formatted to comply with standard industry practice for pharmacy and insurance computer systems.
Store at 20°-25°C (68°-77°F); excursions permitted to 15°-30°C (59°-86°F). [See USP Controlled Room Temperature.]
KEEP THIS AND ALL MEDICATIONS OUT OF THE REACH OF CHILDREN.
-
KEEP THIS AND ALL MEDICATIONS OUT OF THE REACH OF CHILDREN.
All prescription substitutions and/or recommendations using this product shall be made subject to state and federal statutes as applicable. Please note: this is not an Orange Book product and has not been subjected to FDA therapeutic equivalency or other equivalency testing. No representation is made as to generic status or bioequivalency. Each person recommending a prescription substitution using this product shall make such recommendations based on each such person’s professional opinion and knowledge, upon evaluating the dietary ingredients, other ingredients and information provided herein.
THESE STATEMENTS HAVE NOT BEEN EVALUATED BY THE FOOD AND DRUG ADMINISTRATION. THIS PRODUCT IS NOT INTENDED TO DIAGNOSE, TREAT, CURE OR PREVENT ANY DISEASE. - SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 30 Softgel Tablets
-
INGREDIENTS AND APPEARANCE
PNV-OMEGA
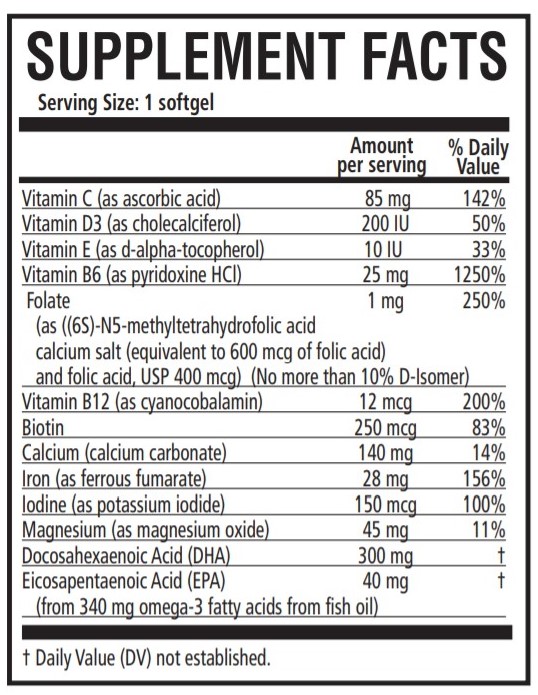
ascorbic acid, cholecalciferol, .alpha.-tocopherol, pyridoxine, folic acid, cyanocobalamin, calcium corbonate, ferrous fumarate, potassium iodide, magnesium, doconexent, icosapent capsule, gelatin coatedProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:42192-332 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ASCORBIC ACID (UNII: PQ6CK8PD0R) (ASCORBIC ACID - UNII:PQ6CK8PD0R) ASCORBIC ACID 85 mg CHOLECALCIFEROL (UNII: 1C6V77QF41) (CHOLECALCIFEROL - UNII:1C6V77QF41) CHOLECALCIFEROL 200 [iU] .ALPHA.-TOCOPHEROL (UNII: H4N855PNZ1) (.ALPHA.-TOCOPHEROL - UNII:H4N855PNZ1) .ALPHA.-TOCOPHEROL 10 [iU] PYRIDOXINE (UNII: KV2JZ1BI6Z) (PYRIDOXINE - UNII:KV2JZ1BI6Z) PYRIDOXINE 25 mg FOLIC ACID (UNII: 935E97BOY8) (FOLIC ACID - UNII:935E97BOY8) FOLIC ACID 1 mg CYANOCOBALAMIN (UNII: P6YC3EG204) (CYANOCOBALAMIN - UNII:P6YC3EG204) CYANOCOBALAMIN 12 ug BIOTIN (UNII: 6SO6U10H04) (BIOTIN - UNII:6SO6U10H04) BIOTIN 250 ug CALCIUM CARBONATE (UNII: H0G9379FGK) (CALCIUM CATION - UNII:2M83C4R6ZB) CALCIUM CATION 140 mg FERROUS FUMARATE (UNII: R5L488RY0Q) (FERROUS CATION - UNII:GW89581OWR) FERROUS CATION 28 mg POTASSIUM IODIDE (UNII: 1C4QK22F9J) (IODIDE ION - UNII:09G4I6V86Q) IODIDE ION 150 ug MAGNESIUM OXIDE (UNII: 3A3U0GI71G) (MAGNESIUM CATION - UNII:T6V3LHY838) MAGNESIUM CATION 45 mg DOCONEXENT (UNII: ZAD9OKH9JC) (DOCONEXENT - UNII:ZAD9OKH9JC) DOCONEXENT 300 mg ICOSAPENT (UNII: AAN7QOV9EA) (ICOSAPENT - UNII:AAN7QOV9EA) ICOSAPENT 40 mg Inactive Ingredients Ingredient Name Strength YELLOW WAX (UNII: 2ZA36H0S2V) CORN OIL (UNII: 8470G57WFM) LECITHIN, SOYBEAN (UNII: 1DI56QDM62) GELATIN (UNII: 2G86QN327L) GLYCERIN (UNII: PDC6A3C0OX) WATER (UNII: 059QF0KO0R) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) FD&C RED NO. 3 (UNII: PN2ZH5LOQY) Product Characteristics Color green Score no score Shape CAPSULE Size 25mm Flavor Imprint Code 332 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:42192-332-30 30 in 1 BOTTLE; Type 0: Not a Combination Product 08/19/2010 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 08/19/2010 Labeler - Acella Pharmaceuticals, LLC (825380939) Establishment Name Address ID/FEI Business Operations Acella Pharmaceuticals, LLC 825380939 manufacture(42192-332)