Label: SYNOVEX CHOICE- trenbolone acetate and estradiol benzoate implant
- NDC Code(s): 54771-3908-1
- Packager: Zoetis Inc.
- Category: OTC ANIMAL DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Animal Drug Application
Drug Label Information
Updated August 9, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
-
DESCRIPTION
SYNOVEX Choice® (trenbolone acetate and estradiol benzoate implants) is a growth promoting implant containing 100 mg of trenbolone acetate and 14 mg of estradiol benzoate per implant. Each implant consists of 4 pellets. Ten implants are provided in each cartridge.
NOTE: Administration of a single SYNOVEX Choice® implant or its use in a reimplantation program with a SYNOVEX Choice®, SYNOVEX Plus®, or SYNOVEX® ONE Feedlot implant may result in decreased marbling scores when compared to non-implanted steers and heifers. -
WITHDRAWAL PERIODS AND RESIDUE WARNINGS
No withdrawal period is required when used according to labeling.
Do not use in beef calves less than 2 months of age, dairy calves, and veal calves. A withdrawal period has not been established for this product in pre-ruminating calves.
Do not use in dairy cows or in animals intended for subsequent breeding. Use in these cattle may cause drug residues in milk and/or in calves born to these cows.
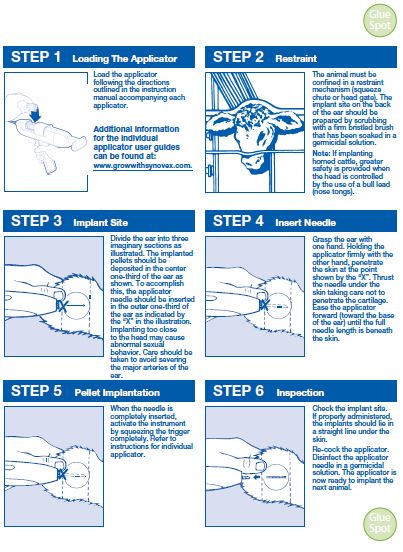
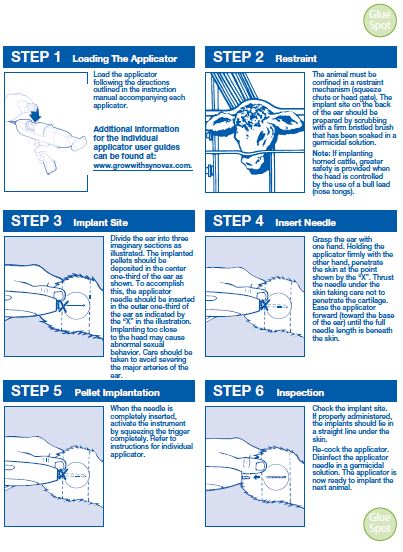
Implant pellets subcutaneously in ear only. Any other location is a violation of Federal law. Do not attempt salvage of implanted site for human or animal food. - USER SAFETY WARNINGS
- ANIMAL SAFETY WARNINGS
-
DIRECTIONS
Administer one SYNOVEX Choice® implant (four pellets), containing 100 mg of trenbolone acetate and 14 mg of estradiol benzoate, to each steer or heifer by subcutaneous implantation in the middle-third of the ear. If using in a reimplantation program, reimplant steers or heifers 60 to 120 days later with a SYNOVEX Choice®, SYNOVEX Plus®, or SYNOVEX® ONE Feedlot implant.
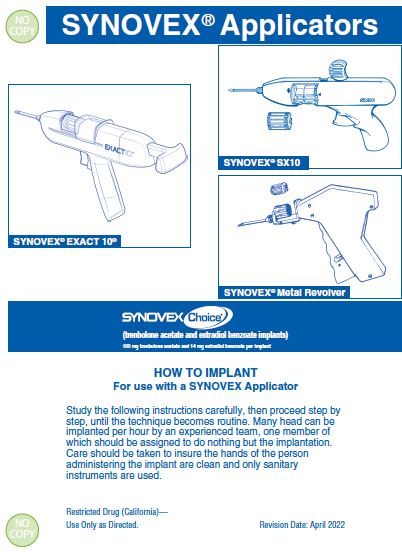
Use only a SYNOVEX applicator. Approved implantation technique is fully described in the fold-out carton section. Never sacrifice careful, clean technique for speed of implantation. -
STORAGE
Store unopened product at controlled room temperature 20°-25°C (68°-77°F) with excursions between 15°-30°C (59°-86°F). Avoid excessive heat and humidity. Use product before the expiration date on the label.
Once the pouch is opened, unused product may be stored in the end-folded pouch (away from light) for up to six months under refrigerated conditions 2°-8°C (36°-47°F) or at room temperature 20°-25°C (68°-77°F) with excursions between 15°-30°C (59°-86°F) for up to one month. - DISPOSAL
-
INDICATIONS FOR USE
•For increased rate of weight gain in growing beef steers fed in confinementfor slaughter and for increased rate of weight gain and improved feedefficiency in growing beef heifers fed in confinement for slaughter.
•For increased rate of weight gain for up to 200 days in growing beef steersand heifers fed in confinement for slaughter in a reimplantation programwhere SYNOVEX Choice® is the first implant and a SYNOVEX Choice®,SYNOVEX Plus®, or SYNOVEX® ONE Feedlot implant is administered60 to 120 days later.
•Other than as described on the labeling, this implant is not approved forrepeated implantation (reimplantation) with any other cattle ear implant ingrowing beef steers and heifers fed in confinement for slaughter as safetyand effectiveness have not been evaluated.
Do not use in beef calves less than 2 months of age, dairy calves, and veal calves because effectiveness and safety have not been established.
Do not use in animals intended for subsequent breeding, or in dairy cows. - QUESTIONS/COMMENTS?
- SPL UNCLASSIFIED SECTION
- IMPORTANT
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 10 Cartridge Implant Carton
-
INGREDIENTS AND APPEARANCE
SYNOVEX CHOICE
trenbolone acetate and estradiol benzoate implantProduct Information Product Type OTC ANIMAL DRUG Item Code (Source) NDC:54771-3908 Route of Administration SUBCUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TRENBOLONE ACETATE (UNII: RUD5Y4SV0S) (TRENBOLONE - UNII:P53R4420TR) TRENBOLONE ACETATE 100 mg ESTRADIOL BENZOATE (UNII: 1S4CJB5ZGN) (ESTRADIOL - UNII:4TI98Z838E) ESTRADIOL BENZOATE 14 mg Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:54771-3908-1 1 in 1 BOX 1 10 in 1 POUCH 1 10 in 1 CARTRIDGE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NADA NADA141043 02/22/1996 Labeler - Zoetis Inc. (828851555)