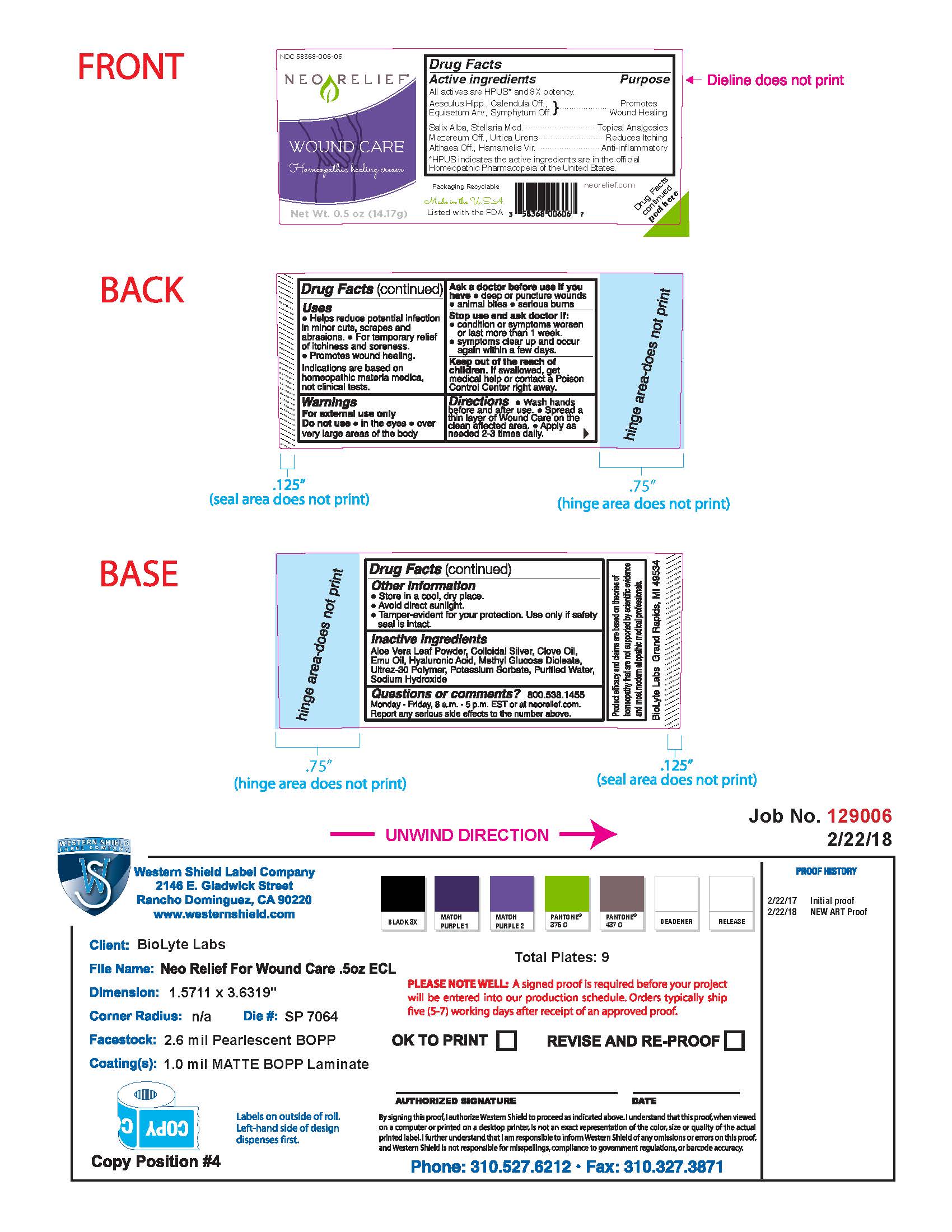
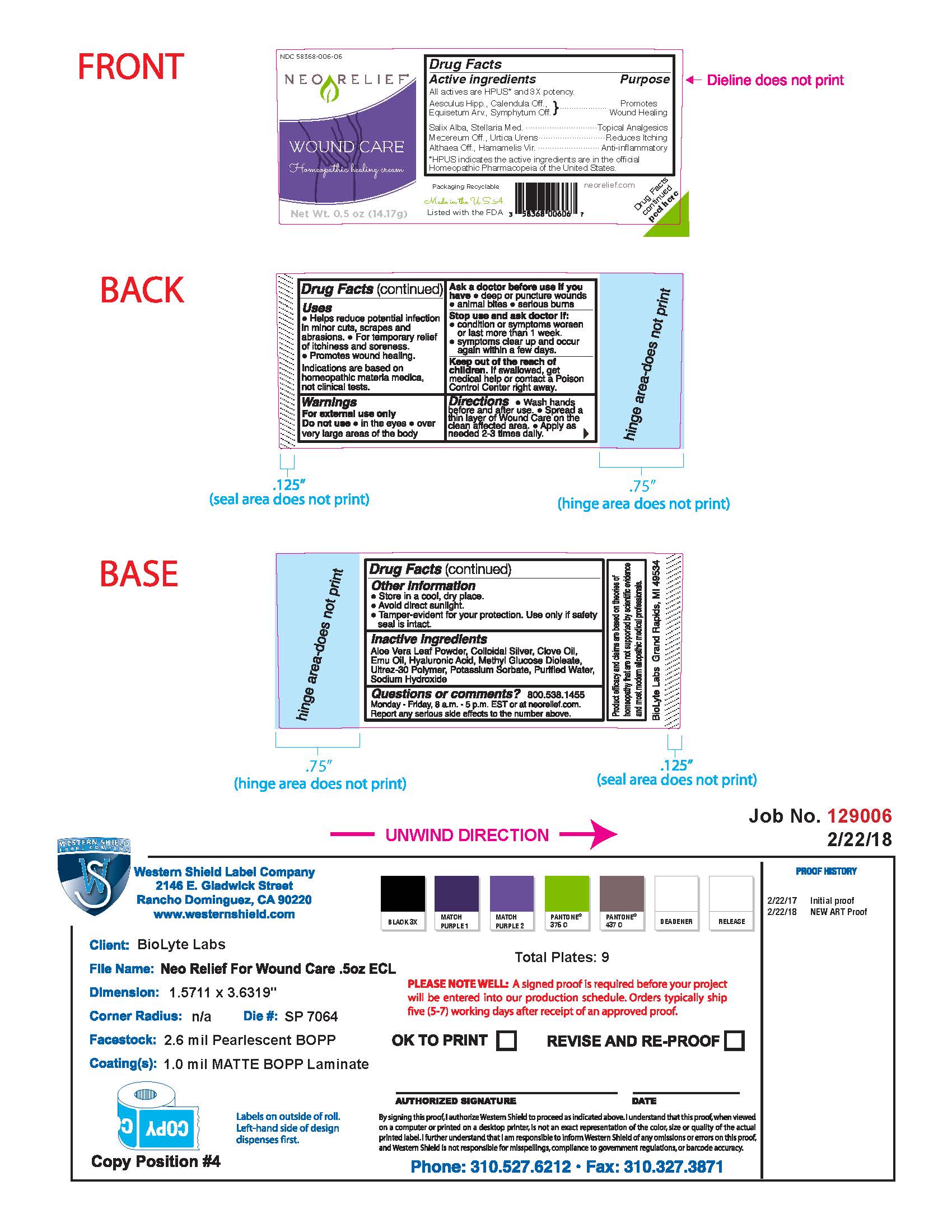
Label: NEORELIEF WOUND CARE- homeopathic healing cream cream
-
Contains inactivated NDC Code(s)
NDC Code(s): 58368-006-06 - Packager: BioLyte Laboratories, LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved homeopathic
DISCLAIMER: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Drug Label Information
Updated November 15, 2018
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts Active Ingredients
- Purpose
- Uses
- Warnings
- Warnings
- Warnings
- Warnings
- Directions
- Other Information
- Inactive Ingredients
- Questions or Comments?
-
Addtional Label Content
Packaging Recyclable
Made in the USA
Listed with the FDA
neorelief.com
Drug Facts peeled here
FTC disclosure: Product efficacy and claims are based on theories of homeopathy that are not supported by scientific evidence and most modern allopathic medical professionals.
BioLyte Labs Grand Rapids, MI 49534
- NEO RELIEF WOUND CARE
-
INGREDIENTS AND APPEARANCE
NEORELIEF WOUND CARE
homeopathic healing cream creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:58368-006 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SALIX ALBA BARK (UNII: 205MXS71H7) (SALIX ALBA BARK - UNII:205MXS71H7) SALIX ALBA BARK 3 [hp_X] in 1 g STELLARIA MEDIA (UNII: 2H03479QVR) (STELLARIA MEDIA - UNII:2H03479QVR) STELLARIA MEDIA 3 [hp_X] in 1 g DAPHNE MEZEREUM BARK (UNII: X2N6E405GV) (DAPHNE MEZEREUM BARK - UNII:X2N6E405GV) DAPHNE MEZEREUM BARK 3 [hp_X] in 1 g URTICA URENS (UNII: IHN2NQ5OF9) (URTICA URENS - UNII:IHN2NQ5OF9) URTICA URENS 2 [hp_X] in 1 g ALTHAEA OFFICINALIS ROOT (UNII: TRW2FUF47H) (ALTHAEA OFFICINALIS ROOT - UNII:TRW2FUF47H) ALTHAEA OFFICINALIS ROOT 3 [hp_X] in 1 g HAMAMELIS VIRGINIANA ROOT BARK/STEM BARK (UNII: T7S323PKJS) (HAMAMELIS VIRGINIANA ROOT BARK/STEM BARK - UNII:T7S323PKJS) HAMAMELIS VIRGINIANA ROOT BARK/STEM BARK 3 [hp_X] in 1 g HORSE CHESTNUT (UNII: 3C18L6RJAZ) (HORSE CHESTNUT - UNII:3C18L6RJAZ) HORSE CHESTNUT 3 [hp_X] in 1 g CALENDULA OFFICINALIS FLOWERING TOP (UNII: 18E7415PXQ) (CALENDULA OFFICINALIS FLOWERING TOP - UNII:18E7415PXQ) CALENDULA OFFICINALIS FLOWERING TOP 3 [hp_X] in 1 g EQUISETUM ARVENSE TOP (UNII: 1DP6Y6B65Z) (EQUISETUM ARVENSE TOP - UNII:1DP6Y6B65Z) EQUISETUM ARVENSE TOP 3 [hp_X] in 1 g Inactive Ingredients Ingredient Name Strength METHYL GLUCOSE DIOLEATE (UNII: FA9KFJ4Z6P) WATER (UNII: 059QF0KO0R) CARBOMER INTERPOLYMER TYPE A (ALLYL SUCROSE CROSSLINKED) (UNII: 59TL3WG5CO) SILVER (UNII: 3M4G523W1G) ALOE VERA LEAF (UNII: ZY81Z83H0X) CLOVE OIL (UNII: 578389D6D0) EMU OIL (UNII: 344821WD61) HYALURONIC ACID (UNII: S270N0TRQY) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) SODIUM HYDROXIDE (UNII: 55X04QC32I) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:58368-006-06 14.17 g in 1 BOTTLE, PUMP; Type 0: Not a Combination Product 04/27/2018 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 04/27/2018 Labeler - BioLyte Laboratories, LLC (015560564) Establishment Name Address ID/FEI Business Operations BioLyte Laboratories, LLC 015560564 manufacture(58368-006) , label(58368-006)