TYLOX
-
acetaminophen and
oxycodone hydrochloride capsule
Ortho-McNeil-Janssen Pharmaceuticals, Inc.
----------
DESCRIPTION
Each capsule of TYLOX (oxycodone and acetaminophen capsules USP) contains:
|
|
| Oxycodone Hydrochloride USP | 5 mg* |
| Warning — May be habit forming. | |
| Acetaminophen USP | 500 mg |
Inactive ingredients: docusate sodium, gelatin, magnesium stearate, sodium benzoate, sodium metabisulfite1, corn starch, FD&C Blue No. 1, FD&C Red No. 3, FD&C Red No. 40, and titanium dioxide.
Acetaminophen occurs as a white, odorless crystalline powder, possessing a slightly bitter taste.
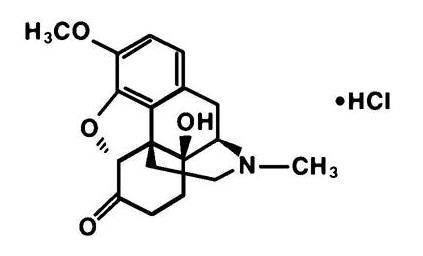
The oxycodone component is 14-hydroxy-dihydrocodeinone, a white, odorless crystalline powder having a saline, bitter taste. It is derived from the opium alkaloid thebaine, and may be represented by the following structural formula:
CLINICAL PHARMACOLOGY
The principal ingredient, oxycodone, is a semi-synthetic narcotic analgesic with multiple actions qualitatively similar to those of morphine; the most prominent of these involve the central nervous system and organs composed of smooth muscle. The principal actions of therapeutic value of the oxycodone in TYLOX (oxycodone and acetaminophen capsules) are analgesia and sedation.
Oxycodone is similar to codeine and methadone in that it retains at least one-half of its analgesic activity when administered orally.
Acetaminophen is a non-opiate, non-salicylate analgesic and antipyretic.
INDICATIONS AND USAGE
TYLOX (oxycodone and acetaminophen capsules) are indicated for the relief of moderate to moderately severe pain.
CONTRAINDICATIONS
TYLOX (oxycodone and acetaminophen capsules) should not be administered to patients who are hypersensitive to any component.
WARNINGS
Contains sodium metabisulfite, a sulfite that may cause allergic-type reactions including anaphylactic symptoms and life-threatening or less severe asthmatic episodes in certain susceptible people. The overall prevalence of sulfite sensitivity in the general population is unknown and probably low. Sulfite sensitivity is seen more frequently in asthmatic than in nonasthmatic people.
Drug Dependence
Oxycodone can produce drug dependence of the morphine type and, therefore, has the potential for being abused. Psychic dependence, physical dependence and tolerance may develop upon repeated administration of TYLOX (oxycodone and acetaminophen capsules), and it should be prescribed and administered with the same degree of caution appropriate to the use of other oral narcotic-containing medications. Like other narcotic-containing medications, TYLOX is subject to the Federal Control Substances Act (Schedule II).
PRECAUTIONS
General
Head Injury and Increased Intracranial Pressure
The respiratory depressant effects of narcotics and their capacity to elevate cerebrospinal fluid pressure may be markedly exaggerated in the presence of head injury, other intracranial lesions or a pre-existing increase in intracranial pressure. Furthermore, narcotics produce adverse reactions which may obscure the clinical course of patients with head injuries.
Information for Patients
Oxycodone may impair the mental and/or physical abilities required for the performance of potentially hazardous tasks such as driving a car or operating machinery. The patient using TYLOX should be cautioned accordingly.
Drug Interactions
Patients receiving other narcotic analgesics, general anesthetics, phenothiazines, other tranquilizers, sedative-hypnotics or other CNS depressants (including alcohol) concomitantly with TYLOX may exhibit an additive CNS depression. When such combined therapy is contemplated, the dose of one or both agents should be reduced.
The concurrent use of anticholinergics with narcotics may produce paralytic ileus.
Usage in Pregnancy
Pregnancy Category C
Animal reproductive studies have not been conducted with TYLOX. It is also not known whether TYLOX can cause fetal harm when administered to a pregnant woman or can affect reproductive capacity. TYLOX should not be given to a pregnant woman unless in the judgment of the physician, the potential benefits outweigh the possible hazards.
Labor and Delivery
As with all narcotics, administration of TYLOX to the mother shortly before delivery may result in some degree of respiratory depression in the newborn and the mother, especially if higher doses are used.
ADVERSE REACTIONS
The most frequently observed adverse reactions include lightheadedness, dizziness, sedation, nausea and vomiting. These effects seem to be more prominent in ambulatory than in non-ambulatory patients, and some of these adverse reactions may be alleviated if the patient lies down.
Other adverse reactions include allergic reactions, euphoria, dysphoria, constipation, skin rash and pruritus. At higher doses, oxycodone has most of the disadvantages of morphine including respiratory depression.
DRUG ABUSE AND DEPENDENCE
TYLOX capsules are a Schedule II controlled substance.
Oxycodone can produce drug dependence and has the potential for being abused. (See WARNINGS)
OVERDOSAGE
Acetaminophen
Signs and Symptoms
In acute acetaminophen overdosage, dose-dependent potentially fatal hepatic necrosis is the most serious adverse effect. Renal tubular necrosis, hypoglycemic coma and thrombocytopenia may also occur.
In adults, hepatic toxicity has rarely been reported with acute overdoses of less than 10 grams and fatalities with less than 15 grams. Importantly, young children seem to be more resistant than adults to the hepatotoxic effect of an acetaminophen overdose. Despite this, the measures outlined below should be initiated in any adult or child suspected of having ingested an acetaminophen overdose.
Early symptoms following a potentially hepatotoxic overdose may include: nausea, vomiting, diaphoresis and general malaise. Clinical and laboratory evidence of hepatic toxicity may not be apparent until 48 to 72 hours post-ingestion.
Treatment
The stomach should be emptied promptly by lavage or by induction of emesis with syrup of ipecac. Patients' estimates of the quantity of a drug ingested are notoriously unreliable. Therefore, if an acetaminophen overdose is suspected, a serum acetaminophen assay should be obtained as early as possible, but no sooner than four hours following ingestion. Liver function studies should be obtained initially and repeated at 24-hour intervals.
The antidote, N-acetylcysteine, should be administered as early as possible, and within 16 hours of the overdose ingestion for optimal results. Following recovery, there are no residual, structural, or functional hepatic abnormalities.
Oxycodone
Signs and Symptoms
Serious overdosage with oxycodone is characterized by respiratory depression (a decrease in respiratory rate and/or tidal volume, Cheyne-Stokes respiration, cyanosis), extreme somnolence progressing to stupor or coma, skeletal muscle flaccidity, cold and clammy skin, and sometimes bradycardia and hypotension. In severe overdosage, apnea, circulatory collapse, cardiac arrest and death may occur.
Treatment
Primary attention should be given to the reestablishment of adequate respiratory exchange through provision of a patent airway and the institution of assisted or controlled ventilation. The narcotic antagonist naloxone hydrochloride is a specific antidote against respiratory depression which may result from overdosage or unusual sensitivity to narcotics, including oxycodone. Therefore, an appropriate dose of naloxone hydrochloride (usual initial adult dose 0.4 mg to 2 mg) should be administered preferably by the intravenous route and simultaneously with efforts at respiratory resuscitation (see package insert). Since the duration of action of oxycodone may exceed that of the antagonist, the patient should be kept under continued surveillance and repeated doses of the antagonist should be administered as needed to maintain adequate respiration.
An antagonist should not be administered in the absence of clinically significant respiratory or cardiovascular depression. Oxygen, intravenous fluids, vasopressors and other supportive measures should be employed as indicated.
Gastric emptying may be useful in removing unabsorbed drug.
DOSAGE AND ADMINISTRATION
Dosage should be adjusted according to the severity of the pain and the response of the patient. However, it should be kept in mind that tolerance to oxycodone can develop with continued use and that the incidence of untoward effects is dose related. This product is inappropriate even in high doses for severe or intractable pain.
TYLOX (oxycodone and acetaminophen capsules) are given orally. The usual adult dosage is one TYLOX capsule every 6 hours as needed for pain.
HOW SUPPLIED
TYLOX (oxycodone and acetaminophen capsules USP): (colored red, imprinted "TYLOX" "McNEIL") NDC 0045-0526-60 – bottles of 100 and NDC-0045-0526-79 - unit dose 100's.
Manufactured by:
Janssen Ortho, LLC
Gurabo, Puerto Rico 00778
Distributed by:
(INSERT OMP LOGO)
OMP DIVISION
ORTHO-McNEIL PHARMACEUTICAL, INC.
Raritan, New Jersey 08869
Revised April 2007
© OMP 2000
7518501
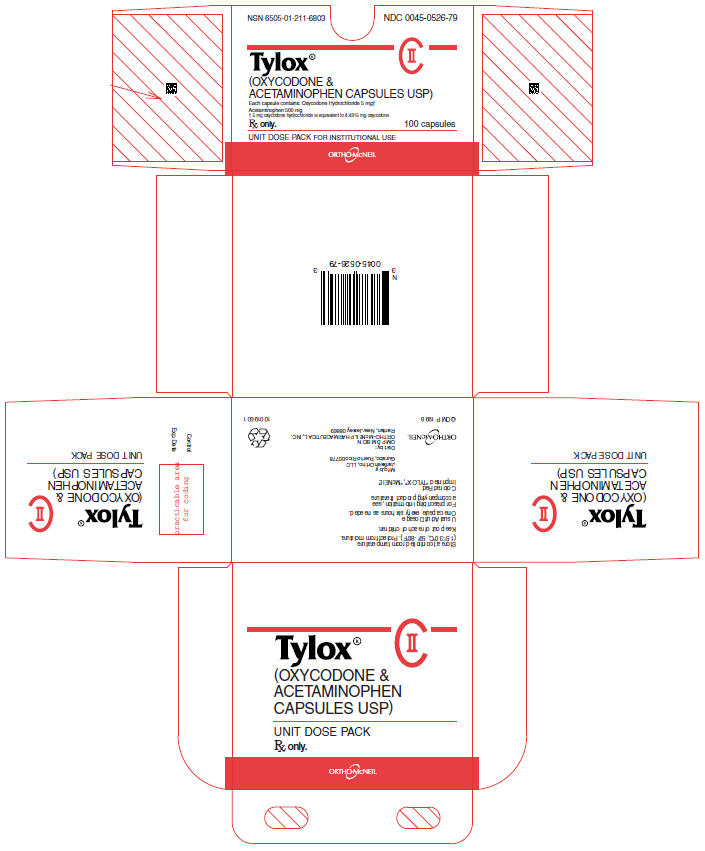
PRINCIPAL DISPLAY PANEL - 100 Capsules Carton
NSN 6505-01-211-6803
NDC 0045-0526-79
Tylox®
(OXYCODONE &
ACETAMINOPHEN CAPSULES USP)
Each capsule contains: Oxycodone Hydrochloride 5 mg†
Acetaminophen 500 mg
† 5 mg oxycodone hydrochloride is equivalent to 4.4815 mg oxycodone.
Rx only.
100 capsules
UNIT DOSE PACK FOR INSTITUTIONAL USE
ORTHO-McNEIL
| TYLOX
acetaminophen and oxycodone hydrochloride capsule |
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| ANDA | ANDA088790 | 12/12/1984 | 08/31/2012 |
| Labeler - Ortho-McNeil-Janssen Pharmaceuticals, Inc. (010779978) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| Janssen Pharmaceuticals, Inc. | 010779978 | MANUFACTURE | |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| Diosite | 409664849 | ANALYSIS, API MANUFACTURE | |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| Ortho-McNeil-Janssen Pharmaceuticals, Inc. | 063137772 | ANALYSIS | |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| Mallinckrodt | 097722284 | ANALYSIS, API MANUFACTURE | |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| Noramco | 057234486 | ANALYSIS | |
Revised: 07/2012 Ortho-McNeil-Janssen Pharmaceuticals, Inc.