BUSINESS ELITE AMENITY- sodium fluoride paste, dentifrice
Hangzhou Zhuwen Inflight & Travel Products Co., Ltd.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Business Elite Amenity Kit
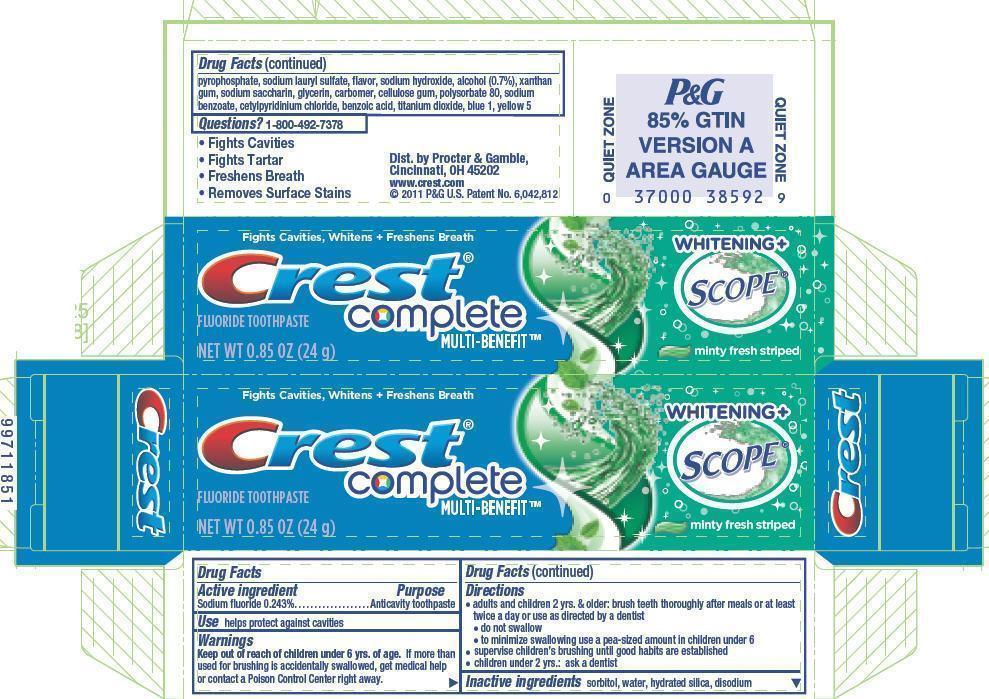
Directions
- adults and children 2 yrs. & older: brush teeth thoroughly after meals or at least twice a day or use as directed by a dentist
- do not swallow
- to minimize swallowing use a pea-sized amount in children under 6
- supervise children's brushing until good habits are established
- children under 2 yrs.: ask a dentist
Inactive ingredients
sorbitol, water, hydrated silica, disodium pyrophosphate, sodium lauryl sulfate, flavor, sodium hydroxide, alcohol (0.7%), xanthan gum, sodium saccharin, glycerin, carbomer, cellulose gum, polysorbate 80, sodium benzoate, cetylpyridinium chloride, benzoic acid, titanium dioxide, blue 1, yellow 5
| BUSINESS ELITE AMENITY
sodium fluoride paste, dentifrice |
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
| Labeler - Hangzhou Zhuwen Inflight & Travel Products Co., Ltd. (421290194) |
| Registrant - Hangzhou Zhuwen Inflight & Travel Products Co., Ltd. (421290194) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Hangzhou Zhuwen Inflight & Travel Products Co., Ltd. | 421290194 | repack(52460-003) , manufacture(52460-003) | |
Revised: 11/2019
Document Id: 97284f6e-b28f-fe4c-e053-2995a90afe80
Set id: 70fe7359-51da-41ac-a013-968626a707fe
Version: 4
Effective Time: 20191112
Hangzhou Zhuwen Inflight & Travel Products Co., Ltd.