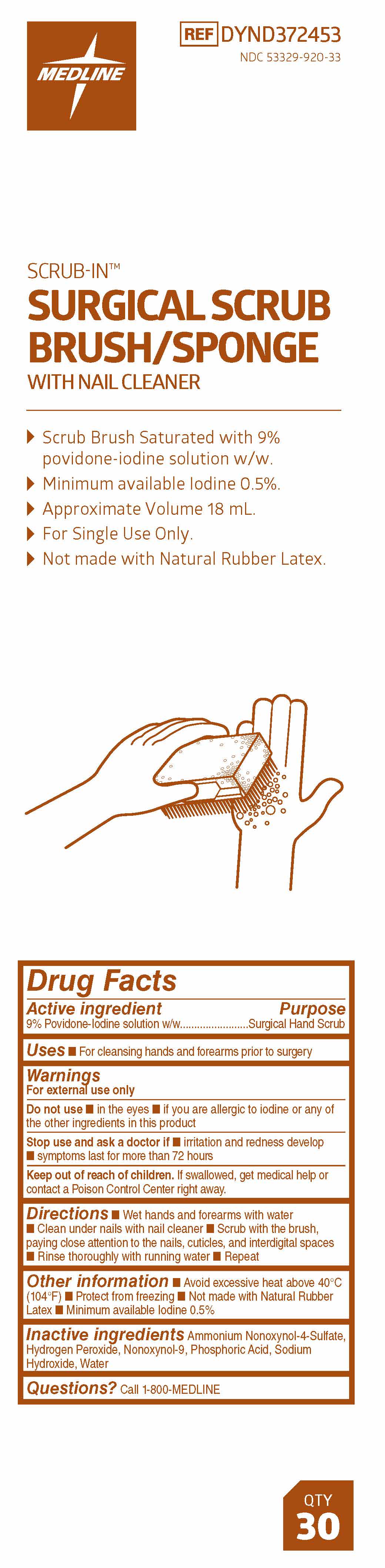
SCRUB-IN SURGICAL SCRUB BRUSH/SPONGE- povidone-iodine solution
Medline Industries, LP
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
920 ScrubIn 9% PVP-I
Warnings
For external use only
Directions
- Wet hands and forearms with water
- Clean under nails with nail cleaner
- Scrub with the brush, paying close attention to the nails, cuticles, and interdigital spaces
- Rinse thoroughly with running water
- Repeat
Other information
- Avoid excessive heat above 40° C (104° F)
- Protect from freezing
- Not made with Natural Rubber Latex
- Minimum available Iodine 0.5%
| SCRUB-IN SURGICAL SCRUB BRUSH/SPONGE
povidone-iodine solution |
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| Labeler - Medline Industries, LP (025460908) |
Revised: 10/2022
Document Id: eb52038b-5033-5169-e053-2995a90adb45
Set id: 70f806e7-0cda-4158-b9c8-3816e9925180
Version: 6
Effective Time: 20221018
Medline Industries, LP