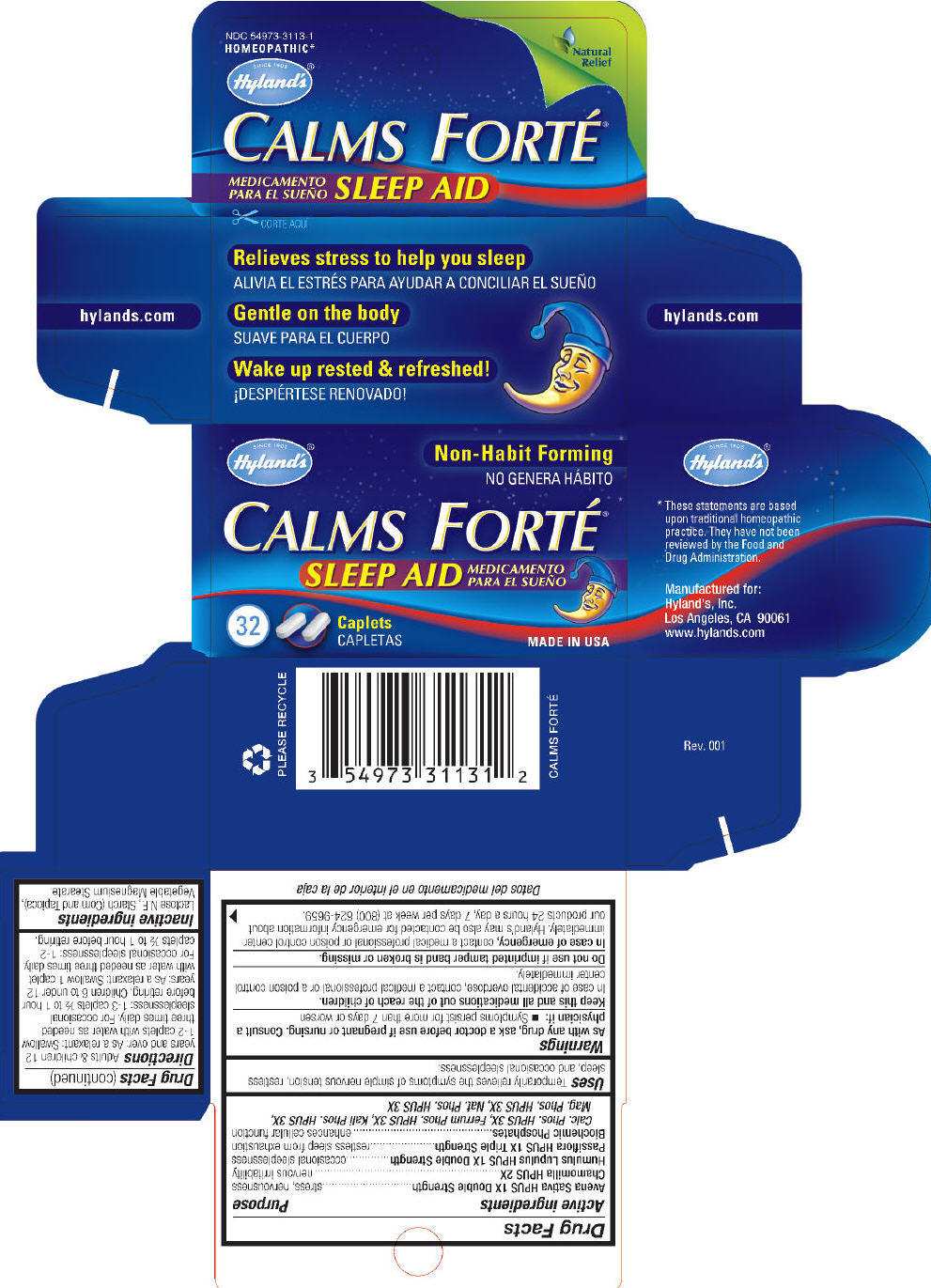
CALMS FORTE- avena sativa flowering top, chamomile, humulus lupulus stem, passiflora incarnata flower, calcium phosphate, ferrosoferric phosphate, potassium phosphate, dibasic, sodium phosphate, and magnesium phosphate, dibasic trihydrate tablet
Hyland's
Disclaimer: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
----------
CALMS FORTE
| Active ingredients | Purpose |
|---|---|
|
Calc. Phos. HPUS 3X, Ferrum Phos. HPUS 3X, Kali Phos. HPUS 3X,
Mag. Phos. HPUS 3X, Nat. Phos. HPUS 3X |
|
| Avena Sativa HPUS 1X Double Strength | stress, nervousness |
| Chamomilla HPUS 2X | nervous irritability |
| Humulus Lupulus HPUS 1X Double Strength | occasional sleeplessness |
| Passiflora HPUS 1X Triple Strength | restless sleep from exhaustion |
| Biochemic Phosphates | enhances cellular function |
Uses
Temporarily relieves the symptoms of simple nervous tension, restless sleep, and occasional sleeplessness.
Warnings
| CALMS FORTE
avena sativa flowering top, chamomile, humulus lupulus stem, passiflora incarnata flower, calcium phosphate, ferrosoferric phosphate, potassium phosphate, dibasic, sodium phosphate, and magnesium phosphate, dibasic trihydrate tablet |
|||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||
| Labeler - Hyland's (028570695) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Standard Homeopathic Company | 008316655 | manufacture(54973-3113) , pack(54973-3113) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Merical, Inc. | 029644978 | manufacture(54973-3113) , pack(54973-3113) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| GMP Laboratories of America, Inc. | 876754375 | manufacture(54973-3113) , pack(54973-3113) | |
Revised: 12/2017
Document Id: 60ced829-a04e-4338-e053-2a91aa0a7900
Set id: 70a3b2aa-566f-4f6a-a400-0d935823dc07
Version: 2
Effective Time: 20171220
Hyland's