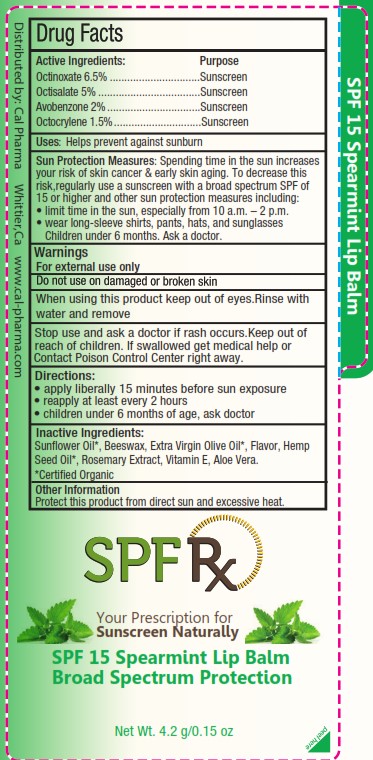
SPF RX - SPF 15 SPEARMINT LIP BALM- octinoxate, octisalate, avobenzone, octocrylene lipstick
Cal Pharma LLC
----------
Cal Pharma (as PLD) - SPF Rx - SPF 15 Spearmint Lip Balm (55628-9575) - DELIST
WARNINGS
For external use only. Do not use on damaged or broken skin. When using this product keep out of eyes. Rinse with water to remove. Stop use and ask a doctor if rash occurs.
DIRECTIONS
Apply liberally 15 minutes before sun exposure. Reapply at least every 2 hours. Children under 6 months, ask a doctor.
Sun Protection Measures: Spending time in the sun increases your risk of skin cancer & early skin aging. To decrease this risk, regularly use a sunscreen with a broad spectrum SPF of 15 or higher and other sun protection measures including:
• limit time in the sun, especially from 10 a.m. - 2 p.m.
• wear long-sleeve shirts, pants, hats, and sunglasses
| SPF RX - SPF 15 SPEARMINT LIP BALM
octinoxate, octisalate, avobenzone, octocrylene lipstick |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Labeler - Cal Pharma LLC (078721283) |