PALLADONE- hydromorphone hydrochloride capsule, extended release
Purdue Pharma LP
----------
PALLADONE
CII
(hydromorphone
hydrochloride extended-release) Capsules
12 mg, 16 mg,
24 mg, 32 mg
WARNING:
Palladone™ (hydromorphone hydrochloride extended-release) Capsules are indicated for the management of persistent, moderate to severe pain in patients requiring continuous, around-the-clock analgesia with a high potency opioid for an extended period of time (weeks to months) or longer. Palladone™ Capsules should only be used in patients who are already receiving opioid therapy, who have demonstrated opioid tolerance, and who require a minimum total daily dose of opiate medication equivalent to 12 mg of oral hydromorphone. Patients considered opioid tolerant are those who are taking at least 60 mg oral morphine/day, or at least 30 mg of oral oxycodone/day, or at least 8 mg oral hydromorphone/day, or an equianalgesic dose of another opioid, for a week or longer. Palladone™ Capsules should be administered once every 24 hours.
Appropriate patients for treatment with Palladone Capsules include patients who require high doses of potent opioids on an around-the-clock basis to improve pain control and patients who have difficulty attaining adequate analgesia with immediate-release opioid formulations.
Palladone Capsules are contraindicated for use on an as needed basis (i.e., prn).
Palladone™ Capsules are NOT intended to be used as the first opioid product prescribed for a patient, or in patients who require opioid analgesia for a short period of time.
Palladone™ Capsules are for use in OPIOID-TOLERANT patients ONLY. Use in non-opioid-tolerant patients may lead to FATAL RESPIRATORY DEPRESSION. Overestimating the Palladone dose when converting patients from another opioid medication can result in fatal overdose with the first dose. Due to the mean apparent 18-hour elimination half-life of Palladone, patients who receive an overdose will require an extended period of monitoring and treatment that may go beyond 18 hours. Even in the face of improvement, continued medical monitoring is required because of the possibility of extended effects.
Palladone™ Capsules contain the potent Schedule II opioid agonist, hydromorphone. Schedule II opioid agonists (which include hydromorphone, fentanyl, methadone, morphine, oxycodone, and oxymorphone), have the highest risk of fatal overdoses due to respiratory depression, as well as the highest potential for abuse. Palladone can be abused in a manner similar to other opioid agonists, legal or illicit. These risks should be considered when administering, prescribing, or dispensing Palladone in situations where the healthcare professional is concerned about increased risk of misuse, abuse, or diversion.
Persons at increased risk for opioid abuse include those with a personal or family history of substance abuse (including drug or alcohol abuse or addiction) or mental illness (e.g., major depression). Patients should be assessed for their clinical risks for opioid abuse or addiction prior to being prescribed opioids. All patients receiving opioids should be routinely monitored for signs of misuse, abuse and addiction. Patients at increased risk of opioid abuse may still be appropriately treated with modified-release opioid formulations; however these patients will require intensive monitoring for signs of misuse, abuse, or addiction.
Palladone capsules are to be swallowed whole and are not to be broken, chewed, opened, dissolved or crushed. Consuming alcohol while taking palladone™ capsules or taking broken, chewed, dissolved, or crushed palladone™ capsules or its contents can lead to the rapid release and absorption of a potentially fatal dose of hydromorphone. Overestimating the palladone dose when converting the patient from another opioid medication can result in fatal overdose with the first dose. With the long half-life of palladone (18 hours), patients who receive the wrong dose will require an extended period of monitoring and treatment that may go beyond 18 hours. Even in the face of improvement, continued medical monitoring is required because of the possibility of extended effects.
DESCRIPTION
Palladone™ (hydromorphone hydrochloride extended-release) Capsules are an opioid analgesic supplied in 12 mg, 16 mg, 24 mg, and 32 mg capsule strengths for oral administration. The pellet formulation is the same for all capsule strengths. The strength designation of each capsule indicates the amount of hydromorphone hydrochloride salt. The structural formula, molecular description, and molecular weight are shown below:
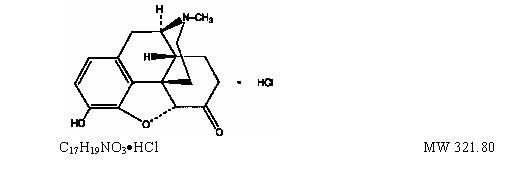 The chemical name is 4,5α-epoxy-3-hydroxy-17
methylmorphinan-6-one hydrochloride.
The chemical name is 4,5α-epoxy-3-hydroxy-17
methylmorphinan-6-one hydrochloride.
Hydromorphone, a fine, white (or nearly white), crystalline powder, is a semi-synthetic congener of morphine. The inactive ingredients in the pellets are ammonio methacrylate copolymer type B, ethylcellulose, and stearyl alcohol. The inactive ingredients in the capsules and the inks used to imprint them are FD&C blue #2 (24 mg strength capsule only), gelatin, red iron oxide (12 mg and 16 mg strength capsules only), synthetic black iron oxide, and titanium dioxide.
Palladone™ Capsules are based on a controlled-release melt extrusion technology in which pellets containing hydromorphone HCl and co-melted excipients release the active ingredient significantly more slowly and for a longer period than an immediate-release product. Palladone™ Capsules are designed to provide controlled delivery of hydromorphone over 24 hours. The 12 mg, 16 mg, 24 mg, and 32 mg capsules are filled with identical pellets using different fill weights to achieve different strengths.
Palladone™ Capsules are for use in opioid-tolerant patients only. Use in non-opioid-tolerant patients may lead to fatal respiratory depression.
CLINICAL PHARMACOLOGY
Hydromorphone is a pure opioid agonist whose principal therapeutic action is analgesia. Other members of the class known as opioid agonists include substances such as morphine, oxycodone, fentanyl, codeine, and hydrocodone. Pharmacological effects of opioid agonists include anxiolysis, euphoria, feelings of relaxation, respiratory depression, constipation, miosis, cough suppression, and analgesia. Like all pure opioid agonist analgesics, with increasing doses there is increasing analgesia, unlike with mixed agonist/antagonists or non-opioid analgesics, where there is a limit to the analgesic effect with increasing doses. With pure opioid agonist analgesics, there is no defined maximum dose; the ceiling to analgesic effectiveness is imposed only by side effects, the more serious of which may include somnolence and respiratory depression.
Central Nervous System
The precise mechanism of the analgesic action is unknown. However, specific CNS opioid receptors for endogenous compounds with opioid-like activity have been identified throughout the brain and spinal cord and play a role in the analgesic effects of this drug.
Hydromorphone produces respiratory depression by direct action of brain stem respiratory centers. The respiratory depression involves both a reduction in the responsiveness of the brain stem to increases in carbon dioxide and to electrical stimulation.
Hydromorphone depresses the cough reflex by direct effect on the cough center in the medulla. Antitussive effects may occur with doses lower than those usually required for analgesia.
Hydromorphone causes miosis even in total darkness. Pinpoint pupils are a sign of opioid overdose but are not pathognomonic (e.g., pontine lesions of hemorrhagic or ischemic origin may produce similar findings). Marked mydriasis rather than miosis may be seen with hypoxia in the setting of Palladone™ Capsule overdose (see OVERDOSAGE).
Gastrointestinal System
Hydromorphone causes a reduction in motility associated with an increase in smooth muscle tone in the antrum of the stomach and in the duodenum. Digestion of food is delayed in the small intestine and propulsive contractions are decreased. Propulsive peristaltic waves in the colon are decreased, while tone may be increased to the point of spasm resulting in constipation. Other opioid induced-effects may include a reduction in gastric, biliary and pancreatic secretions, spasm of the sphincter of Oddi, and transient elevations in serum amylase.
Cardiovascular System
Hydromorphone may produce release of histamine with or without associated peripheral vasodilation. Manifestations of histamine release and/or peripheral vasodilation may include pruritus, flushing, red eyes, sweating, and/or orthostatic hypotension.
Endocrine System
Opioid agonists have been shown to have a variety of effects on the secretion of hormones. Opioids inhibit the secretion of ACTH, cortisol, and luteinizing hormone (LH) in humans. They also stimulate prolactin, growth hormone (GH) secretion, and pancreatic secretion of insulin and glucagon in humans and other species, rats and dogs. Thyroid stimulating hormone (TSH) has been shown to be both inhibited and stimulated by opioids.
PHARMACOKINETICS
Absorption
Administration of a single Palladone™ Capsule dose is characterized by biphasic absorption, a relatively rapid rise to an initial peak concentration, followed by a second broader peak with therapeutic plasma concentrations maintained over the 24-hour dosing interval. The absolute bioavailability of hydromorphone from Palladone™ Capsules has not been determined. Under conditions of multiple dosing, the bioavailability of a once-daily dose of Palladone™ Capsules is equivalent to the same total daily dose of immediate-release hydromorphone given in divided doses every 6 hours. Hydromorphone absorption from Palladone Capsules is pH independent but can be significantly increased in the presence of alcohol (see PHARMACOKINETICS: Drug Interactions). Dose proportionality has been established in terms of Cmax and AUC for the 12 mg and 24 mg dosage strengths. Dosage form proportionality on a dose-adjusted basis has been demonstrated for three 12 mg capsules to one 32 mg capsule.
In a study comparing 12 mg Palladone™ Capsules dosed every 24 hours to 3 mg of immediate-release hydromorphone dosed every 6 hours in healthy human subjects, the two treatments were found to be equivalent in terms of extent of absorption (AUC) (see Figure 1). The extended-release characteristics of Palladone™ Capsules resulted in lower steady-state peak levels (Cmax), higher trough levels (Cmin), and an approximately twofold to threefold reduction in the fluctuation seen with the immediate-release hydromorphone tablets.
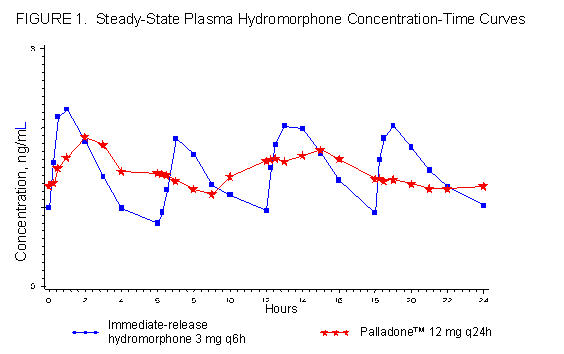
Steady-state plasma concentrations with Palladone™ Capsules were achieved within 2 to 3 days after initiation of dosing. This is consistent with the mean apparent terminal elimination half-life for Palladone™ of approximately 18.6 hours. This supports the ability to titrate every 2 to 3 days, as necessary. Hydromorphone did not accumulate significantly after multiple dosing with once-daily administration.
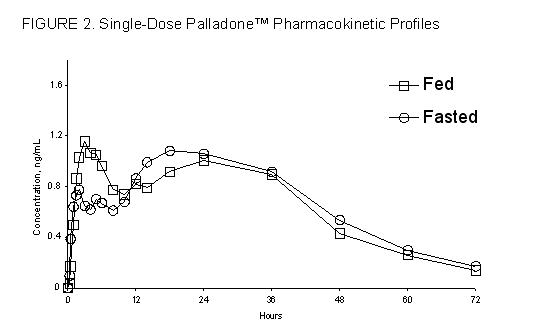
Food had no significant effect on the peak (Cmax), AUC or the elimination of hydromorphone from Palladone™ Capsules (See Figure 2.).
Distribution
Following intravenous admininstration of hydromorphone, the reported volume of distribution is 295 L (4 L/kg). Hydromorphone is approximately 20% bound to human plasma proteins.
Metabolism
Hydromorphone is metabolized by direct conjugation, or by 6-keto reduction followed by conjugation. Following absorption, hydromorphone is metabolized to the major metabolites hydromorphone-3-glucuronide, hydromorphone-3-glucoside and dihydroisomorphine-6-glucuronide. Also observed were the less prevalent metabolites, dihydroisomorphine-6-glucoside, dihydromorphine and dihydroisomorphine.
Hydromorphone metabolites have been found in plasma, urine and in human hepatocyte test systems. However, it is not known whether hydromorphone is metabolized by the cytochrome P450 enzyme system. Hydromorphone is a poor inhibitor of human recombinant CYP isoforms including CYP1A2, 2A6, 2C8, 2D6, and 3A4 with an IC50 > 50 µM. Therefore, hydromorphone is not expected to inhibit the metabolism of other drugs metabolized by these CYP isoforms.
Elimination
Full mass balance and recovery studies have not been reported for extended-release hydromorphone products. However, hydromorphone and its metabolites have been recovered in urine following the use of immediate-release hydromorphone. Following intravenous administration of hydromorphone, terminal half-life is approximately 3 hours and clearance is 1.66 L/hr. The apparent terminal half-life with controlled release hydromorphone is about 18.6 hours.
Drug Interactions
Concomitant administration of H2 receptor blockers (cimetidine, famotidine, ranitidine) or proton pump inhibitors (omeprazole, lansoprazole) showed no significant effect on Palladone™ steady-state pharmacokinetics.
Patients taking Palladone with other opioid analgesics, general anesthetics, phenothiazines, tricyclic antidepressants or other CNS depressants may experience additional CNS depression and therefore, dose adjustments should be considered. Consuming alcohol while taking Palladone Capsules can cause significant increases in peak hydromorphone concentrations.
SPECIAL POPULATIONS
Pediatric
The safety and effectiveness of Palladone™ Capsules have not been established in patients below the age of 18.
Geriatric
Age-related increases in exposure in clinical studies were observed between geriatric and younger adult subjects. Greater sensitivity of some older individuals cannot be excluded. Dosages should be adjusted according to the clinical situation.
Renal Impairment
In patients with mild to moderate renal impairment, based on calculated creatinine clearance, the concentrations of hydromorphone in plasma were slightly higher than in subjects with normal renal function.
CLINICAL TRIALS
The efficacy of Palladone™ Capsules was established in a double-blind, randomized, parallel group, multicenter, placebo-controlled, four-week trial of patients with pain that was present for at least one month. The majority of these patients experienced moderate to severe pain due to musculoskeletal disorders while maintained on one or more opioid analgesics, often in addition to non-opioid analgesics. Two hundred twenty-one patients with chronic moderate to severe pain were randomized to receive once daily 12 mg Palladone™ Capsules or placebo after they had demonstrated that they needed approximately 12 mg of immediate-release hydromorphone (in addition to non-opioid medication) around-the-clock to improve their pain control. Patients randomized to Palladone™ Capsules maintained adequate analgesia for a significantly longer period of time (P<0.0001) than patients randomized to placebo.
INDICATIONS AND USAGE
Palladone™ Capsules are indicated for the management of persistent, moderate to severe pain in patients requiring continuous, around-the-clock analgesia with a high potency opioid for an extended period of time generally weeks to months or longer. Palladone™ Capsules should only be used in patients who are already receiving opioid therapy, have demonstrated opioid tolerance, and who require a minimum total daily dose of opiate medication equivalent to 12 mg of oral hydromorphone. Patients considered opioid tolerant are those who are taking at least 60 mg oral morphine/day, or at least 30 mg oral oxycodone/day, or at least 8 mg oral hydromorphone/day, or an equianalgesic dose of another opioid, for a week or longer. Appropriate patients for treatment with Palladone include patients who require high doses of potent opioids on an around-the-clock basis to improve pain control, and patients who have difficulty attaining adequate analgesia with immediate-release opioid formulations.
Palladone™ Capsules are NOT intended to be used:
- as the first opioid product prescribed for a patient.
- in patients who require opioid analgesia for a short period of time.
- on an as needed basis (i.e., prn).
An evaluation of the appropriateness and adequacy of immediate-release opioids is advisable prior to initiating therapy with any modified-release opioid. Prescribers should individualize treatment in every case, initiating therapy at the appropriate point along a progression from non-opioid analgesics, such as non-steroidal anti-inflammatory drugs and acetaminophen, to opioids, in a plan of pain management such as outlined by the World Health Organization, the Agency for Health Research and Quality, the Federation of State Medical Boards Model Policy, or the American Pain Society.
Patients should be assessed for their clinical risks for opioid abuse or addiction prior to being prescribed opioids. Patients receiving opioids should be routinely monitored for signs of misuse, abuse, and addiction. Persons at increased risk for opioid abuse include those with a personal or family history of substance abuse (including drug or alcohol abuse or addiction) or mental illness (e.g., major depression). Patients at increased risk may still be appropriately treated with modified-release opioid formulations; however these patients will require intensive monitoring for signs of misuse, abuse, or addiction.
CONTRAINDICATIONS
Palladone™ Capsules are contraindicated:
- for use on an as needed basis (i.e. prn).
- in situations of significant respiratory depression, especially in unmonitored settings where there is a lack of resuscitative equipment.
- in patients who have acute or severe bronchial asthma.
- in patients who have or are suspected of having paralytic ileus.
- in patients with known hypersensitivity to any of its components or the active ingredient, hydromorphone.
WARNINGS
Palladone Capsules are to be swallowed WHOLE and are not to be broken, chewed, OPENED, DISSOLVED OR CRUSHED. Consuming alcohol while taking Palladone Capsules or taking broken, chewed, dissolved, or crushed Palladone™ Capsules or capsule contents can lead to the rapid release and absorption of a potentially fatal dose of hydromorphone.
Palladone™ Capsules are for use in OPIOID-TOLERANT patients ONLY. Use in non-opioid-tolerant patients may lead to fatal respiratory depression.
Misuse, Abuse and Diversion of Opioids
Hydromorphone is an opioid agonist of the morphine type. Such drugs are sought by drug abusers and people with addiction disorders and are subject to criminal diversion.
Like other opioid agonists, legal or illicit, hydromorphone can be abused. This should be considered when prescribing or dispensing Palladone™ Capsules in situations where the healthcare professional is concerned about an increased risk of misuse, abuse, or diversion (see WARNINGS: Drug Abuse and Addiction).
Breaking, crushing, chewing, or dissolving the contents of a Palladone™ Capsule or consuming alcohol while taking Palladone Capsules can result in the uncontrolled delivery of the opioid and poses a significant risk of overdose and death (see Boxed WARNING).
Concerns about abuse, addiction, and diversion should not prevent the proper management of pain. However, all patients treated with opioids require careful monitoring for signs of abuse and addiction, since use of opioid analgesic products carries the risk of addiction even under appropriate medical use.
Healthcare professionals should contact their State Professional Licensing Board, or State Controlled Substances Authority for information on how to prevent and detect abuse or diversion of this product.
Interactions with Alcohol and Drugs of Abuse
Hydromorphone may be expected to have additive effects, when used in conjunction with alcohol, other opioids, or drugs, whether legal or illicit, which cause central nervous system depression. Additionally, consuming alcohol while taking Palladone Capsules can cause significant increases in peak hydromorphone concentrations.
Drug Abuse and Addiction
Palladone™ Capsules contain an opioid agonist (i.e., hydromorphone), that is a Schedule II controlled substance with high potential for abuse similar to fentanyl, methadone, morphine, oxycodone, and oxymorphone. Hydromorphone can be abused and is subject to criminal diversion. The high drug content in the extended-release formulation may add to the risk of adverse outcomes from abuse.
Addiction is a primary, chronic, neurobiologic disease, with genetic, psychosocial, and environmental factors influencing its development and manifestations. It is characterized by behaviors that include one or more of the following: impaired control over drug use, compulsive use, continued use despite harm, and craving. Drug addiction is a treatable disease, utilizing a multidisciplinary approach, but relapse is common.
“Drug-seeking” behavior is very common in addicts and drug abusers. Drug-seeking tactics include emergency calls or visits near the end of office hours, refusal to undergo appropriate examination, testing or referral, repeated “loss” of prescriptions, tampering with, forging or counterfeiting prescriptions and reluctance to provide prior medical records or contact information for other treating physician(s). “Doctor shopping” to obtain additional prescriptions is common among drug abusers, people suffering from untreated addiction and criminals seeking drugs to sell.
Abuse and addiction are separate and distinct from physical dependence and tolerance. Physicians should be aware that addiction may not be accompanied by concurrent tolerance and symptoms of physical dependence in all addicts. In addition, abuse of opioids can occur in the absence of addiction and is characterized by misuse for non-medical purposes, often in combination with other psychoactive substances. Since Palladone™ Capsules may be diverted for non-medical use, careful record keeping of prescribing information, including quantity, frequency, and renewal requests is strongly advised.
Proper assessment of the patient, proper prescribing practices, periodic re-evaluation of therapy, and proper dispensing and storage are appropriate measures that help to limit abuse of opioid drugs.
Palladone™ Capsules are intended for oral use only. Consumption of alcohol while taking Palladone Capsules or the use of the broken, crushed, chewed, opened, or dissolved capsule contents poses a hazard of overdose and death. This risk is increased with concurrent abuse of alcohol and other substances. Parenteral drug abuse can reasonably be expected to result in local tissue necrosis, infection, pulmonary granulomas, and increased risk of endocarditis and valvular heart injury. In addition, parenteral abuse is commonly associated with transmission of infectious diseases such as hepatitis and HIV.
Respiratory Depression
Respiratory depression is the chief hazard of opioid agonists, including hydromorphone, the active ingredient in Palladone™ Capsules. Respiratory depression is more likely to occur in elderly or debilitated patients, usually following large initial doses in non-tolerant patients, or when opioids are given in conjunction with other drugs that depress respiration.
Respiratory depression from opioids is manifested by a reduced urge to breathe and a decreased rate of respiration, often associated with the “sighing” pattern of breathing (deep breaths separated by abnormally long pauses). Carbon dioxide retention from opioid-induced respiratory depression can exacerbate the sedating effects of opioids. This makes overdoses involving drugs with sedative properties and opioids especially dangerous.
Hydromorphone should be used with extreme caution in patients with significant chronic obstructive pulmonary disease or cor pulmonale, and in patients having a substantially decreased respiratory reserve, hypoxia, hypercapnia, or pre-existing respiratory depression. In such patients, even usual therapeutic doses of hydromorphone may decrease respiratory drive to the point of apnea. In these patients, alternative non-opioid analgesics should be considered, and opioids should be employed only under careful medical supervision at the lowest effective dose.
Head Injury
The respiratory depressant effects of opioids include carbon dioxide retention and secondary elevation of cerebrospinal fluid pressure and may be markedly exaggerated in the presence of head injury, intracranial lesions, or other sources of pre-existing increased intracranial pressure. Hydromorphone produces effects on pupillary response and consciousness, which may obscure neurologic signs of further increases in intracranial pressure in patients with head injuries.
Hypotensive Effect
Palladone™ Capsules may cause severe hypotension. There is an added risk to individuals whose ability to maintain blood pressure has been compromised by a depleted blood volume or who are concurrently taking drugs such as phenothiazines or other agents which compromise vasomotor tone. Hydromorphone may produce orthostatic hypotension in ambulatory patients. Hydromorphone, like all opioid analgesics of the morphine-type, should be administered with caution to patients in circulatory shock, since vasodilation produced by the drug may further reduce cardiac output and blood pressure.
PRECAUTIONS
Palladone™ Capsules are for use in OPIOID-TOLERANT patients ONLY. Use in non-opioid-tolerant patients may lead to fatal respiratory depression (see WARNINGS).
Patients should be instructed that the use of Palladone™ by anyone other than those to whom it is prescribed is unlawful and may have serious medical consequences, including death.
General
Opioid analgesics have a narrow therapeutic index in certain patient populations, especially when combined with CNS depressant drugs, and should be reserved for cases where the benefits of opioid analgesia outweigh the known risks of respiratory depression, altered mental state, and postural hypotension.
Use of Palladone™ Capsules is associated with increased potential risks and should be used only with caution in the following conditions: alcoholism, alcohol abuse or alcohol intoxication; drug abuse; history of drug or alcohol abuse; adrenocortical insufficiency (e.g., Addison's disease); CNS depression or coma; debilitated patients; kyphoscoliosis associated with respiratory depression; myxedema or hypothyroidism; prostatic hypertrophy or urethral stricture; severe impairment of hepatic, pulmonary or renal function; and toxic psychosis.
The administration of any opioid agonist, including hydromorphone, may obscure the diagnosis or clinical course in patients with acute abdominal conditions. Hydromorphone may aggravate convulsions in patients with convulsive disorders, and all opioids may induce or aggravate seizures in some clinical settings.
Use in Pancreatic/Biliary Tract Disease
Hydromorphone may cause spasm of the sphincter of Oddi and should be used with caution in patients with biliary tract disease, including acute pancreatitis. Opioids like hydromorphone may cause increases in the serum amylase concentration.
Tolerance
Tolerance is a state of adaptation in which exposure to a drug induces changes that result in a diminution of one or more of the drug’s effects over time. Tolerance may occur to both the desired and undesired effects of drugs, and may develop at different rates for different effects.
Physical Dependence
Physical dependence is a state of adaptation that is manifested by an opioid specific withdrawal syndrome that can be produced by abrupt cessation, rapid dose reduction, decreasing blood level of the drug, and/or administration of an antagonist.
The opioid abstinence or withdrawal syndrome is characterized by some or all of the following: restlessness, lacrimation, rhinorrhea, yawning, perspiration, chills, piloerection, myalgia, mydriasis, irritability, anxiety, backache, joint pain, weakness, abdominal cramps, insomnia, nausea, anorexia, vomiting, diarrhea, or increased blood pressure, respiratory rate, or heart rate.
In general, opioids should not be abruptly discontinued (see DOSAGE AND ADMINISTRATION: Cessation of Therapy).
Information for Patients/Caregivers
The healthcare professional should explain the points listed below to caregivers and patients.
- Patients should be instructed to read the Medication Guide each time Palladone is dispensed because new information may be available. The complete text of the Medication Guide is reprinted at the end of this document.
- Patients should be aware that Palladone™ Capsules contain hydromorphone, which is a strong pain medication similar to fentanyl, methadone, morphine, oxycodone, and oxymorphone.
- Palladone™ Capsules are only to be swallowed whole. To prevent fatal overdose, the capsules and the pellets must not be broken, chewed, crushed, opened, or dissolved. Patients should be instructed not to consume alcohol while taking Palladone Capsules.
- Patients should talk to their doctor if pain persists or worsens while they are taking Palladone™ Capsules. Patients who have bothersome side effects should also let their doctors know. The amount of medicine the patient takes may have to be changed.
- Patients should NEVER change the amount of Palladone™ Capsules they take without speaking to their doctor first.
- Palladone™ Capsules can affect a person’s ability to perform activities that require a high level of attention (such as driving or using heavy machinery). Patients taking Palladone™ Capsules should be warned of these dangers and counseled accordingly.
- Patients should NOT combine Palladone™ Capsules with alcohol or other pain medications, sleep aids, or tranquilizers except by the orders of the prescribing physician, because dangerous additive effects may occur, resulting in serious injury or death.
- Women who become pregnant, or who plan to become pregnant, should ask their doctor about the effects that Palladone™ Capsules (or any medicine) may have on them and their unborn children.
- Patients should be advised that if they have been receiving treatment with Palladone™ Capsules for more than a few weeks and the medicine is no longer needed, they should contact their doctor who will advise them on how to gradually decrease the medication. When Palladone™ Capsules are no longer needed, the unused capsules should be flushed down the toilet.
- The active ingredient in Palladone™ Capsules is hydromorphone, which is a drug that some people abuse. Palladone™ Capsules should be taken only by the patient it was prescribed for, and it should be protected from theft or misuse in the work or home environment.
- Patients should be instructed to keep Palladone™ Capsules in a secure place out of the reach of children. Children, especially small children, exposed to Palladone™ Capsules are at high risk of FATAL RESPIRATORY DEPRESSION.
Use in Drug and Alcohol Addiction
Palladone™ Capsules are not approved for use in detoxification or maintenance treatment of opioid addiction. However, the history of an addictive disorder does not necessarily preclude the use of this medication for the treatment of chronic pain. These patients will require intensive monitoring for signs of misuse, abuse, or addiction.
DRUG INTERACTIONS
CNS Depressants
Hydromorphone should be dosed with caution in patients who are concurrently taking other central nervous system depressants that may cause respiratory depression, hypotension, profound sedation or potentially result in coma. Such agents include barbiturates, other sedatives or hypnotics, general anesthetics, other opioid analgesics, phenothiazines and other neuroleptics, centrally acting anti-emetics, benzodiazepines or other tranquilizers, and alcohol.
Muscle Relaxants
Hydromorphone may interact with skeletal muscle relaxants to enhance neuromuscular blocking action to increase respiratory depression.
Mixed Agonist-Antagonist Opioid Analgesics
Agonist/antagonist analgesics (i.e., pentazocine, nalbuphine, and butorphanol) should be administered with caution to a patient who has received or is receiving a course of therapy with a pure opioid agonist analgesic such as hydromorphone. In this situation, significant doses of mixed agonist/antagonist analgesics may reduce the analgesic effect of hydromorphone and/or may precipitate withdrawal symptoms in these patients.
Monoamine Oxidase Inhibitors (MAOIs)
No specific interaction between hydromorphone and monoamine oxidase inhibitors has been observed, but caution in the use of any opioid in patients taking this class of drugs is appropriate. MAOI therapy should be discontinued for at least two weeks prior to the initiation of therapy with Palladone™ Capsules.
H2 Antagonists/Proton Pump Inhibitors
In the patients enrolled in the clinical trials, Palladone™ exposure and effects on pain were comparable when administered with or without various H2 antagonists/proton pump inhibitors.
Drug/Laboratory Test Interactions
There is no known interference of this drug with laboratory tests.
Carcinogenesis, Mutagenesis, Impairment of Fertility
No carcinogenicity studies have been conducted in animals.
Hydromorphone was negative in the in vitro bacterial reverse mutation assay and in the in vivo mouse micronucleus assay. Hydromorphone was negative in the mouse lymphoma assay in the absence of metabolic activation, but was positive in the mouse lymphoma assay in the presence of metabolic activation. Morphinone, an impurity, tested as a besylate salt was negative in the in vitro bacterial reverse mutation assay and negative in the in vivo mouse micronucleus assay. Morphinone was positive in the Chinese Hamster Ovary Cell Chromosomal Aberration test in the absence and presence of metabolic activation.
Hydromorphone did not affect fertility in rats at oral doses up to 5 mg/kg which is equivalent to a 32 mg human daily oral dose on a body surface area basis.
Pregnancy
Pregnancy Category C
Hydromorphone was not teratogenic in female rats given oral doses up to 10 mg/kg or female rabbits given oral doses up to 50 mg/kg during the major period of organ development. Estimated exposures in the female rat and rabbit were approximately 3-fold and 6-fold higher than a 32 mg human daily oral dose based on exposure (AUC0-24h). In a rat pre- and post-natal study, an increase in pup mortality and a decrease in pup body weight which was associated with maternal toxicity was observed at doses of 2 and 5 mg/kg/day. The maternal no effect level for hydromorphone was 0.5 mg/kg/day which is <1-fold lower than a 32 mg human daily oral dose on a body surface area basis. Hydromorphone had no effect on pup development or reproduction when given to female rats during the pre-natal and postnatal periods up to a dose of 5 mg/kg which is equivalent to a 32 mg human daily oral dose on a body surface area basis.
Hydromorphone administration to pregnant Syrian hamsters and CF-1 mice during major organ development revealed teratogenicity likely the result of maternal toxicity associated with sedation and hypoxia. In Syrian hamsters given single subcutaneous doses from 14 to 278 mg/kg during organogenesis (gestation days 8-10), doses ≥ 19 mg/kg hydromorphone produced skull malformations (exencephaly and cranioschisis). Continuous infusion of hydromorphone (5 mg/kg, s.c.) via implanted osmotic mini pumps during organogenesis (gestation days 7-10) produced soft tissue malformations (cryptorchidism, cleft palate, malformed ventricals and retina), and skeletal variations (supraoccipital, checkerboard and split sternebrae, delayed ossification of the paws and ectopic ossification sites). The malformations and variations observed in the hamsters and mice were at doses approximately 3-fold higher and <1-fold lower, respectively, than a 32 mg human daily oral dose on a body surface area basis.
There are no adequate and well-controlled studies in pregnant women. Hydromorphone crosses the placenta. Palladone™ Capsules should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus (see Labor and Delivery).
Labor and Delivery
Palladone™ Capsules are not recommended to be initiated prior to or during labor or in the immediate post-partum period. Women who are taking opioids during pregnancy should not be withdrawn abruptly during labor and delivery, but maintained on their current dose of medication since abrupt withdrawal can precipitate delivery. Neonates whose mothers have been taking hydromorphone chronically may exhibit respiratory depression and/or withdrawal symptoms, at birth and/or in the post-delivery period.
Neonatal Withdrawal Syndrome
Chronic use of opioids during pregnancy can affect the fetus with subsequent withdrawal symptoms. Neonatal withdrawal syndrome presents as irritability, hyperactivity and loss of sleep pattern, abnormal crying, tremor, vomiting, diarrhea and subsequent weight loss or failure to gain weight and may result in death. The duration and severity of neonatal withdrawal syndrome varies based on the drug used, duration of use, the time and dose of last maternal use, and rate of elimination by the newborn. Use standard care as medically appropriate.
Nursing Mothers
Low concentrations of opioid analgesics have been detected in breast milk with the potential for withdrawal symptoms when administration of opioid analgesics to the mother is stopped. The distribution of hydromorphone has not been studied. It is prudent to assume that hydromorphone would also distribute into breast milk. Ordinarily, nursing should not be undertaken while a patient is receiving Palladone™ Capsules because of the possibility of sedation and/or respiratory depression in the infant.
Pediatric Use
The safety and effectiveness of Palladone™ Capsules have not been established in patients below the age of 18 years.
Geriatric Use
Of the total number of subjects in clinical studies of Palladone™ Capsules, 22% were 65 and over, and 6% were 75 and over. Dosages should be adjusted according to the clinical situation. As with all opioids, the starting dose should be reduced to 1/3 to 1/2 of the usual dosage in debilitated patients. Respiratory depression is the chief hazard in elderly or debilitated patients, usually following large initial doses in non-tolerant patients, or when opioids are given in conjunction with other agents that depress respiration.
Laboratory Monitoring
Due to the broad range of plasma concentrations that are associated with individual daily dose requirements to achieve adequate pain relief, the varying degrees of pain, and the development of tolerance seen in patient populations, plasma hydromorphone measurements are usually not helpful in clinical management.
Hepatic Impairment
Palladone™ Capsules were not studied in patients with severe hepatic impairment and are not recommended for use in such patients. Care in initial dose selection and careful observation are recommended in patients with evidence of mild to moderate hepatic impairment.
Renal Impairment
In patients with mild to moderate renal impairment, based on calculated creatinine clearance, the concentrations of hydromorphone in plasma were slightly higher than in subjects with normal renal function. Dosages should always be adjusted according to the clinical situation.
ADVERSE REACTIONS
The safety of Palladone™ Capsules was evaluated in double-blind clinical trials involving 612 patients with moderate to severe pain. An open-label extension study involving 143 patients with cancer pain was conducted to evaluate the safety of Palladone™ Capsules when used for longer periods of time in higher doses than in the controlled trials. Patients were treated with doses averaging 40 to 50 mg of Palladone™ Capsules per day (ranging between 12 and 500 mg/day) for several months (range 1 to ≥52 weeks).
Serious adverse reactions which may be associated with Palladone™ Capsules therapy in clinical use are similar to those of other opioid analgesics, including respiratory depression, apnea, respiratory arrest, and to a lesser degree, circulatory depression, hypotension, shock or cardiac arrest (see OVERDOSAGE).
Adverse Events Reported in Controlled Trials
Table 3 lists treatment emergent signs and symptoms that were reported in at least 2% of patients in the placebo-controlled trials for which the rate of occurrence was greater for those treated with 12mg Palladone™ Capsules than those treated with placebo.drug abuse and dependence (addiction)
| Placebo* (N = 191) | Palladone™* (N = 190) |
|
|---|---|---|
| Body System/ COSTART Term | Double-blind
% | Double-blind
% |
| * Average exposure was 21 days for Palladoneä and 15 days for placebo. | ||
| Total percentage of patients with AEs | 35.1% | 49.5% |
| Body as a Whole | 15.7% | 18.4% |
| Headache Asthenia Infection | 2.1% 0.5% 5.8% | 4.7% 3.2% 5.3% |
| Digestive | 13.1% | 27.9% |
| Constipation Nausea Vomiting | 1.0% 6.3% 1.6% | 15.8% 10.5% 3.2% |
| Nervous | 13.1% | 11.6% |
| Somnolence | 1.6% | 4.7% |
| Skin | 5.2% | 4.7% |
| Pruritus | 1.0% | 2.6% |
Adverse Events Observed in Clinical Trials
Palladone™ Capsules have been administered to 785 individuals during completed clinical trials. The conditions and duration of exposure to Palladone™ varied greatly, and included open-label and double-blind studies, uncontrolled and controlled studies, inpatient and outpatient studies, fixed dose and titration studies. Untoward events associated with this exposure were recorded by clinical investigators using terminology of their own choosing.
These categories are used in the listing below. The frequencies represent the proportion of 785 patients from these trials who experienced that event while receiving Palladone™ Capsules. All adverse events included in this tabulation occurred in at least one patient. Events are classified by body system and listed using the following definitions: frequent adverse events - those occurring in at least 1/100 patients; adverse events occurring with an incidence less than 1% are considered infrequent. These adverse events are not necessarily related to Palladone™ Capsule treatment and in most cases were observed at a similar frequency in placebo-treated patients in the controlled studies.
Frequent Adverse Events
Body as a Whole: headache, asthenia, pain, abdominal pain, fever, chest pain, infection, chills, malaise, neck pain, carcinoma, accidental injury
Cardiovascular: vasodilatation, tachycardia, migraine
Digestive: nausea, constipation, vomiting, diarrhea, dyspepsia, anorexia, dry mouth, nausea and vomiting, dysphagia, flatulence
Hematologic and Lymphatic: anemia, leukopenia
Metabolic and Nutritional: peripheral edema, dehydration, edema, generalized edema, hypokalemia, weight loss
Musculoskeletal: arthralgia, bone pain, leg cramps, myalgia
Nervous: somnolence, dizziness, nervousness, confusion, insomnia, anxiety, depression, hypertonia, hypesthesia, paresthesia, tremor, thinking abnormal, hallucinations, speech disorder, agitation, amnesia, tinnitus, abnormal gait
Respiratory: dyspnea, cough increased, rhinitis, pharyngitis, pneumonia, epistaxis, hiccup, hypoxia, pleural effusion
Skin and Appendages: pruritus, sweating, rash
Special Senses: amblyopia, taste perversion
Urogenital: dysuria, urinary incontinence Infrequent Adverse Events
Infrequent Adverse Events
Body as a Whole: face edema, ascites, allergic reaction, cellulitis, overdose, hypothermia, neoplasm, photosensitivity reaction, sepsis, flank pain
Cardiovascular: hypertension, hypotension, syncope, deep thrombophlebitis, arrhythmia, postural hypotension, atrial fibrillation, pallor, bradycardia, electrocardiogram abnormal, myocardial infarction, palpitation, angina pectoris, congestive heart failure, QT interval prolonged, supraventricular tachycardia, thrombosis, cardiomegaly, hemorrhage
Digestive: fecal impaction, intestinal obstruction, abnormal stools, fecal incontinence, hepatic failure, increased appetite, cholangitis, cholecystitis, colitis, enterocolitis, hepatomegaly, jaundice, liver function tests abnormal, biliary spasm, ileus, eructation, rectal hemorrhage, esophagitis, glossitis, melena, mouth ulceration, gastrointestinal hemorrhage, tongue edema
Endocrine: adrenal cortex insufficiency
Hematologic and Lymphatic: ecchymosis, thrombocytopenia, leukocytosis, lymphadenopathy, agranulocytosis, lymphoma like reaction, pancytopenia, petechia
Metabolic and Nutritional: hyperglycemia, hyponatremia, cachexia, hypercalcemia, hypomagnesemia, cyanosis, diabetes mellitus, gout, respiratory acidosis, elevated liver enzymes, thirst
Nervous: abnormal dreams, emotional lability, paranoid reaction, sleep disorder euphoria, incoordination, stupor, ataxia, convulsion, hallucination, hostility, myoclonus, psychosis, vertigo, withdrawal syndrome, apathy, delirium, dementia, drug dependence, nystagmus, twitching, depersonalization, aphasia, cerebrovascular accident, circumoral parasthesia, seizure, hyperkinesia, hypotonia, increased salivation, neuralgia
Respiratory: hypoventilation, apnea, atelectasis, hemoptysis, asthma, hyperventilation, pulmonary embolus, laryngismus
Skin and Appendages: urticaria, maculopapular rash, alopecia
Special Senses: abnormal vision, diplopia, dry eyes, lacrimation disorder, hyperacusis
Urogenital: urinary retention, hematuria, impotence, urinary frequency, urination impaired, dysmenorrhea, creatinine increased, urinary urgency
OVERDOSAGE
Acute overdosage with hydromorphone can be manifested by respiratory depression, somnolence progressing to stupor or coma, skeletal muscle flaccidity, cold and clammy skin, constricted pupils, bradycardia, hypotension, and death.
The nature of extended-release hydromorphone should also be taken into account when treating the overdose. Even in the face of improvement, continued medical monitoring is required because of the possibility of extended effects. Deaths due to overdose may occur with abuse and misuse of Palladone™ Capsules.
In the treatment of hydromorphone overdosage, primary attention should be given to the re-establishment of a patent airway and institution of assisted or controlled ventilation. Supportive measures (including oxygen and vasopressors) should be employed in the management of circulatory shock and pulmonary edema accompanying overdose as indicated. Cardiac arrest or arrhythmias may require cardiac massage or defibrillation.
The pure opioid antagonists, such as naloxone or nalmefene, are specific antidotes against respiratory depression from opioid overdose. Opioid antagonists should not be administered in the absence of clinically significant respiratory or circulatory depression secondary to hydromorphone overdose. In patients who are physically dependent on any opioid agonist including Palladone™ Capsules, an abrupt or complete reversal of opioid effects may precipitate an acute abstinence syndrome. The severity of the withdrawal syndrome produced will depend on the degree of physical dependence and the dose of the antagonist administered. Please see the prescribing information for the specific opioid antagonist for details of their proper use.
DOSAGE AND ADMINISTRATION
Special Precautions
Palladone™ Capsules contain the potent Schedule II opioid agonist, hydromorphone. Schedule II opioid agonists, which include hydromorphone, fentanyl, methadone, morphine, oxycodone, and oxymorphone, have the highest risk of fatal overdoses from respiratory depression, as well as the highest potential for abuse. Hydromorphone, like morphine and other opioids used in analgesia, can be abused and is subject to criminal diversion.
Consuming alcohol while taking Palladone Capsules or taking broken, chewed, dissolved or crushed Palladone™ Capsules or its contents can lead to the rapid release and absorption of a potentially fatal dose of hydromorphone.
Overestimating the Palladone dose when converting patients from another opioid medication can result in fatal overdose with the first dose. Due to the mean apparent 18-hour elimination half-life of Palladone, patients who receive an overdose will require an extended period of monitoring and treatment that may go beyond 18 hours. Even in the face of improvement, continued medical monitoring is required because of the possibility of extended effects.
Palladone™ Capsules are for use in OPIOID-TOLERANT patients only. Use in non-opioid-tolerant patients may lead to FATAL RESPIRATORY DEPRESSION.
General Principles
Palladone™ Capsules are indicated for the management of persistent, moderate to severe pain in patients requiring continuous, around-the-clock analgesia with a high potency opioid for an extended period of time, generally weeks to months, or longer.
Palladone™ Capsules should only be used in patients who are already receiving opioid therapy, who have demonstrated opioid tolerance, and who require a minimum total daily dose of opiate medication equivalent to 12 mg of oral hydromorphone. Patients considered opioid tolerant are those who are taking at least 60 mg oral morphine/day, or at least 30 mg oral oxycodone/day, or at least 8 mg oral hydromorphone/day, or an equianalgesic dose of another opioid, for a week or longer. It is usually appropriate to treat a patient with only one opioid for around-the-clock therapy.
Palladone™ Capsules are not intended to be used on an as needed basis or as the first opioid product prescribed for a patient, or in patients who require opioid analgesia for a short period of time.
The extended-release nature of the formulation allows it to be administered once every 24 hours (see CLINICAL PHARMACOLOGY).
Physicians should individualize treatment using a progressive plan of pain management such as outlined by the World Health Organization, the American Pain Society and the Federation of State Medical Boards Model Policy. Healthcare professionals should follow appropriate pain management principles of careful assessment and ongoing monitoring (see BOXED WARNING).
Initiation of Therapy
Palladone™ Capsules are for use in opioid-tolerant patients ONLY.
It is critical to initiate the dosing regimen individually for each patient, taking into account the patient’s prior opioid treatment. Overestimating the Palladone dose when converting patients from another opioid medication can result in fatal overdose with the first dose. Due to the mean apparent 18-hour elimination half-life of Palladone, patients who receive an overdose will require an extended period of monitoring and treatment that may go beyond 18 hours. Even in the face of improvement, continued medical monitoring is required because of the possibility of extended effects.
- the patient’s medical condition, past medical history, co-morbid conditions and concurrent medications;
- risk factors for abuse, addiction or diversion, including a prior history of abuse, addiction or diversion;
- the intensity, pattern, quality and expected duration of pain;
- the daily dose, potency and kind of opioid the patient has been taking;
- whether adjustments should be made to any non-opioid analgesic(s) the patient has been taking;
- the clinical variability of any conversion estimate used to calculate the dose of hydromorphone; and
- the balance between an adequate level of pain control and adverse experiences.
The total daily (24-hour) dose of the patient’s previous opioid should be determined.
-
Using standard conversion ratio estimates (see Table 4 below), multiply the milligrams per day of the previous opioids by the appropriate conversion factors to obtain the equivalent total daily dose of oral hydromorphone. The conversion ratios represent a reasonable starting point, although they have not been verified in well-controlled, multiple-dose trials.
-
Modify the dose if indicated, based primarily on considerations outlined in the 7 bulleted items listed above.
-
Round off to a dose which is appropriate for the capsule strengths available (12 mg, 16 mg, 24 mg, and 32 mg capsules).
-
Discontinue all other around-the-clock opioid analgesics when Palladone™ Capsules are initiated.
No fixed conversion ratio is likely to be satisfactory in all patients, especially patients receiving large opioid doses. The recommended doses are only a starting point, and close observation and frequent titration is indicated until a satisfactory dose is obtained on the new therapy.
| Factor
PRIOR OPIOID | ORAL | PARENTERAL |
|---|---|---|
| * To be used only for conversion TO oral hydromorphone. For patients receiving high-dose parenteral opioids, a more conservative conversion is warranted. For example, for high dose parenteral morphine, use 0.38 instead of 0.75 as a multiplication factor, i.e., halve the multiplication factor. | ||
| Codeine | 0.04 | – |
| Hydrocodone | 0.22 | – |
| Hydromorphone | 1.00 | 5.00 |
| Levorphanol | 1.88 | 3.75 |
| Meperidine | 0.02 | 0.10 |
| Methadone | 0.38 | 0.75 |
| Morphine | 0.12 | 0.75 |
| Oxycodone | 0.25 | – |
Most patients given around-the-clock therapy with controlled-release opioids may need to have immediate-release medication available for exacerbations of pain or to treat or prevent pain that occurs predictably during certain patient activities (incident pain).
Palladone™ Capsules can be safely used concomitantly with usual doses of non-opioid analgesics and analgesic adjuvants, provided care is taken to select a proper initial dose (see PRECAUTIONS).
Conversion from Transdermal Fentanyl to Palladone™ Capsules
Eighteen hours following the removal of the transdermal fentanyl patch, Palladone™ Capsule treatment can be initiated. A conservative hydromorphone dose, approximately 12 mg once a day of Palladone™, should be initially substituted for each 50 µg/hr of transdermal fentanyl. The patient should be observed closely for early titration as there is very limited clinical experience with this conversion.
Conversion from Opioid Combination Drugs
Patients currently receiving around-the-clock fixed-combination-opioid analgesics, and greater than or equal to a daily dose of 45 mg of oxycodone or hydrocodone equivalents or 300 mg daily dose of codeine equivalents, may be started on 12 mg of Palladone™ Capsules once daily. The non-opioid component of the combination product may be continued as a separate drug. Alternatively, a different non-opioid analgesic may be selected.
Supplemental (Rescue) Analgesics
Most patients given around-the-clock therapy with controlled-release opioids may need to have immediate-release medication available for exacerbations of pain or to treat or prevent pain that occurs predictably during certain patient activities (incident pain).
Individualization of Dosage
Once therapy is initiated, pain relief and other opioid effects should be assessed frequently. Palladone™ Capsules should be titrated to adequate effect (generally mild or less pain with the regular use of no more than two doses of supplemental analgesics per 24 hours). Rescue medication should be available (see Supplemental [Rescue] Analgesics). Because steady-state plasma concentrations are achieved within approximately 2 to 3 days of therapy with Palladone™, dosage adjustment can be carried out as frequently as every two days, when clinically necessary. If more than two doses of rescue medication are needed within a 24 hour period for two consecutive days, the dose of Palladone™ should usually be titrated upward. This formulation should be administered once every 24 hours. As a guideline, the total daily hydromorphone dose (including rescue) usually can be increased by 25% to 50% of the current dose at each upward titration.
If signs of excessive opioid-related adverse experiences are observed, the dose may be reduced. If this adjustment leads to inadequate analgesia, a supplemental dose of immediate-release opioid analgesic may be given. Alternatively, non-opioid analgesic adjuvants may be administered. Dose adjustments should be made to obtain an appropriate balance between pain relief and opioid-related adverse experiences.
If common opioid-related adverse events occur before the therapeutic goal of pain relief is achieved, the events should be effectively treated prior to continuing upward titration of Palladone™ Capsules. Once adverse events are under control, upward titration should continue to an acceptable level of pain control.
During periods of changing analgesic requirements, including initial titration, frequent contact is recommended between physician, other members of the healthcare team, the patient and the caregiver/family.
Managing Expected Opioid Adverse Reactions
Many patients receiving opioids will experience adverse reactions. Frequently the adverse reactions from Palladone™ Capsules are transient, but often they require evaluation and management. Certain opioid adverse reactions such as constipation should be anticipated and effectively and prophylactically treated with a stimulant laxative and/or stool softener. Patients do not usually become tolerant to the constipating effects of opioids.
Other opioid-related adverse reactions such as sedation and nausea are usually self-limited and often do not persist beyond the first few days. If nausea persists and is unacceptable to the patient, treatment with antiemetics or other modalities may relieve these symptoms and should be considered.
Continuation of Therapy
The intent of the titration period is to establish a patient-specific daily dose that will provide adequate analgesia with acceptable side effects and minimal rescue doses (2 or less) for as long as pain relief is necessary. Should pain recur, the dose can be increased to re-establish pain control. The method of therapy adjustment outlined above should be employed to regain pain control.
During chronic around-the-clock opioid therapy, patients should be followed closely and their pain should be reassessed as clinically indicated. Patients should continue to be assessed for their clinical risks for opioid abuse or addiction, particularly with high-dose formulations.
SPECIAL HANDLING AND STORAGE CONDITIONS
Palladone™ Capsules are solid dosage forms that contain hydromorphone which is a controlled substance. Like fentanyl, methadone, morphine, oxycodone, and oxymorphone, hydromorphone is controlled under Schedule II of the Federal Controlled Substances Act.
Palladone™ Capsules may be targeted for theft and diversion by criminals.
Healthcare professionals can telephone Purdue Pharma's Medical Services Department (1-888-726-7535) for information on this product.
HOW SUPPLIED
Palladone™ (hydromorphone hydrochloride extended-release) Capsules 12 mg are cinnamon-colored capsules imprinted with P-XL on the cap and 12 mg on the body. They are available in multiple-use containers, not intended for dispensing directly to the patient. They are supplied as follows:
NDC 59011-312-60: opaque plastic
bottles of 60 capsules
NDC 59011-312-20:
Unit dose packaging with 20 capsules; 10 individually numbered blister
units per card: two cards per carton
Palladone™ (hydromorphone hydrochloride extended-release) Capsules 16 mg are pink capsules imprinted with P-XL on the cap and 16 mg on the body. They are available in multiple-use containers, not intended for dispensing directly to the patient. They are supplied as follows:
NDC 59011-313-60: opaque plastic bottles of 60 capsules
NDC 59011-313-20: Unit dose packaging with 20 capsules;
10 individually numbered blister units per card; two cards per carton
Palladone™ (hydromorphone hydrochloride extended-release) Capsules 24 mg are blue capsules imprinted with P-XL on the cap and 24 mg on the body. They are available in multiple-use containers, not intended for dispensing directly to the patient. They are supplied as follows:
NDC 59011-314-60: opaque plastic
bottles of 60 capsules
NDC 59011-314-20:
Unit dose packaging with 20 capsules; 10 individually numbered blister
units per card; two cards per carton
Palladone™ (hydromorphone hydrochloride extended-release) Capsules 32 mg are white capsules imprinted with P-XL on the cap and 32 mg on the body. They are available in multiple-use containers, not intended for dispensing directly to the patient. They are -supplied as follows:
NDC 59011-315-60: opaque plastic bottles of 60 capsules
NDC 59011-315-20: Unit dose packaging with 20 capsules;
10 individually numbered blister units per card; two cards per carton
Store at 25°C (77°F); excursions permitted to 15°-30°C (59°-86°F) [See USP Controlled Room Temperature].
Avoid temperatures above 40°C (104°F) [See USP Excessive Heat]
Copyright © 2004, Purdue Pharma
L.P.
Purdue Pharma L.P.
Stamford,
CT 06901-3431, USA
11-Feb-2005
U.S. Patent Numbers 5,958,452; 5,965,161; 5,968,551;
6,294,195;
6,335,033; 6,706,281; 6,743,442.
OT00470E
301179-0A
MEDICATION GUIDE
PALLADONE™ (PAL-ah-doan)
(hydromorphone hydrochloride extended-release) Capsules CII
12 mg, 16 mg, 24 mg, 32 mg
Read the Medication Guide that comes with Palladone™ before you start taking it and each time you get more Palladone. There may be new information. This information does not take the place of talking to your healthcare provider about your medical condition or your treatment. Share this important information with members of your household.
What is the most important information I should know about Palladone™?
- Palladone is only for adults with constant (around the clock) pain that is moderate to severe and expected to last for weeks or longer. Palladone should only be started if you are already using other narcotic medicines and your body has gotten used to them (opioid tolerant). Palladone can cause serious side effects, including trouble breathing, which can lead to death, especially if used the wrong way.
- Palladone is not for occasional ("as needed") use.
- Palladone should not be the first opioid (narcotic) pain medicine that is prescribed for your pain.
- Palladone is not for patients who need opioid pain medicines for only a short time.
- Do not drink alcohol while taking Palladone Capsules. Do not break, crush, dissolve, chew, or open Palladone™ Capsules. Palladone Capsules must be swallowed whole. Drinking alcohol while taking Palladone Capsules or taking a broken, crushed, dissolved, or chewed Palladone Capsule or its contents can release the full 24-hour dose into your body all at once. This is very dangerous. You could die from an overdose of the medicine.
- Keep Palladone in a safe place away from children. Accidental use by a child is a medical emergency and can result in death. If a child accidentally takes Palladone, call your local Poison Control Center or the nearest emergency room right away.
- Palladone is an opioid (narcotic) pain medicine. There is a chance you could get addicted to Palladone. The chance is higher if you are or have been addicted to or abused other medicines, street drugs, or alcohol, or if you have a history of mental problems.
- Palladone™ is a Schedule II, federally controlled substance because it contains an opioid (narcotic) pain medicine that can be a target for people who abuse prescription medicines or street drugs. Keep your Palladone in a safe place to protect it from being stolen. Never give Palladone to anyone else, even if they have the same symptoms you have. It may harm them and cause death. Selling or giving away this medicine is against the law.
What is Palladone™?
Palladone™ is a prescription medicine that
contains the opioid (narcotic) pain medicine hydromorphone. Palladone™
is a very strong pain medicine. Palladone is used to treat adults
(18 years of age and older) with constant (around-the-clock) pain that is moderate to severe and
is expected to last for weeks or longer. Palladone should be started
only after you have been taking other opioid pain medicines and your
body has gotten used to them (opioid tolerant). You must stay under
your healthcare provider’s care while taking Palladone.
Palladone Capsules are not to be used:
- as the first opioid pain medicine prescribed for you
- if you only need opioid pain medicine for a short time
- for occasional (“as needed”) use
Who should not take Palladone™?
- your pain can be taken care of by occasional use of other pain medicines.
- you have acute (sudden) or severe asthma
- you have a stomach problem called a paralytic ileus
- you are allergic to any of the ingredients in Palladone. The active ingredient is hydromorphone. For a complete list of ingredients, see “What are the ingredients of Palladone?” at the end of this leaflet.
What should I tell
my healthcare provider before starting Palladone?
Tell your healthcare provider about all of your medical and mental
problems, especially the ones listed below:
- trouble breathing or lung problems such as asthma, wheezing, or shortness of breath
- a head injury
- liver or kidney problems
- seizures (convulsions or fits)
- gallbladder problems
- low thyroid (hypothyroidism)
- low blood pressure
- problems urinating
- mental problems including major depression or hallucinations (seeing or hearing things that are not there)
- adrenal gland problems such as Addison’s disease
- a past or present drinking problem or alcoholism, or a family history of this problem
- a past or present drug abuse or addiction problem, or a family history of this problem
Tell your healthcare provider if you are:
- pregnant or planning to become pregnant. Palladone may harm your unborn baby.
- breast feeding. Palladone likely passes through your milk and it may cause serious harm to your baby. You and your doctor should decide whether you should take Palladone or breastfeed, but not both.
Tell your healthcare provider about all the medicines you take, including prescription and non-prescription medicines, vitamins, and herbal supplements. Some medicines may cause serious or life-threatening medical problems when taken with Palladone™. Sometimes, the doses of certain medicines and Palladone need to changed if used together. Be especially careful about other medicines, sleeping pills, anxiety medicines, antihistamines, or tranquilizers.
Do not start any new prescription medicine, non-prescription medicine, or herbal supplement while using Palladone until you have talked to your healthcare provider. Your healthcare provider will tell you if it is safe to take other medicines while you are using Palladone.
- Take Palladone exactly as prescribed.
- Palladone Capsules must be swallowed whole with water. If you cannot swallow the capsule whole, tell your healthcare provider who will advise you what to do. Do not break, chew, dissolve, crush, or open Palladone Capsules or their contents before swallowing. Drinking alcohol while taking Palladone Capsules or taking a broken, chewed, dissolved, or crushed Palladone Capsule or its contents can release the full 24-hour dose into your body all at once. This is very dangerous. You could die from an overdose of the medicine.
- Your healthcare provider may change your dose after seeing how the medicine affects you.
- Do not change your dose unless your healthcare provider tells you to change it.
- Do not take Palladone™ more often than prescribed.
- Take Palladone once a day at the same time every day.
- If you miss a dose, take it as soon as possible. Take your next dose 24 hours later. Do not double your prescribed dose of Palladone at any time because this increases your chance of an overdose. If you are not sure what to do, call your healthcare provider.
- If you take too much Palladone or overdose, call your local emergency number or Poison Control Center right away, or get emergency help.
- Talk to your healthcare provider often about your pain. Your healthcare provider can decide if your dose of Palladone needs to be changed.
If you continue to have pain or side effects that worry you, call your healthcare provider.
Stopping Palladone™. You should not suddenly stop taking Palladone™. Palladone can cause physical dependence. If your healthcare provider decides you no longer need Palladone™, ask how to slowly reduce this medicine so you don’t get sick with withdrawal symptoms. Do not stop taking Palladone without talking to your healthcare provider. Stopping Palladone suddenly can make you sick with withdrawal symptoms because your body has become used to it. After stopping Palladone according to the instructions of your healthcare provider, flush the unused capsules down the toilet.
What should I avoid while taking Palladone™?
- Do not drive, operate heavy machinery, or do other dangerous activities until you know how Palladone affects how alert you are. Palladone™ can make you sleepy. Ask your healthcare provider when it is okay to do these activities.
- Do not drink alcohol while using Palladone. It may increase your chance of getting dangerous side effects.
- Do not take other medicines without talking to your healthcare provider. Other medicines include prescription and non-prescription medicines, vitamins, and herbal supplements. Be especially careful about medicines that make you sleepy such as other pain medicines, sleeping pills, anxiety medicines, antihistamines, and tranquilizers.
- Do not breast feed while using Palladone. Palladone likely passes through your milk and it may cause serious harm to your baby. You and your doctor should decide whether you should take Palladone or breastfeed, but not both.
What are the possible or reasonably likely side effects of Palladone™?
Palladone can cause serious side effects including death, especially if used the wrong way. See “What is the most important information I should know about Palladone?”
Call your healthcare provider or get emergency medical help if you:
- have trouble breathing
- have extreme drowsiness with slowed breathing
- have shallow breathing (little chest movement with breathing)
- feel faint, dizzy, confused, or have other unusual symptoms
These can be symptoms that you have taken too much (overdose) Palladone or the dose is too high for you. These symptoms may lead to serious problems or death if not treated right away.
- Palladone can cause your blood pressure to drop. This can make you feel dizzy if you get up too fast from sitting or lying down.
- You can develop physical dependence on Palladone. Stopping Palladone suddenly can make you sick with withdrawal symptoms because your body has become used to it. Talk to your healthcare provider about slowly stopping Palladone.
- There is a chance you could get addicted to Palladone. The chance is higher if you are or have been addicted to or abused other medicines, street drugs, or alcohol, or if you have a history of mental problems.
The common side effects of Palladone™ are constipation, nausea, vomiting, nervousness, dizziness, drowsiness, itching, dry mouth, sweating, weakness, and headache. Constipation (not enough or hard bowel movements) is a very common side effect of opioids including Palladone and is unlikely to go away without treatment. Talk to your healthcare provider about the use of laxatives (medicines to treat constipation) and stool softeners to prevent or treat constipation while taking Palladone.
Talk to your healthcare provider about any side effect that bothers you or that does not go away.
These are not all the possible side effects of Palladone™. For a complete list, ask your healthcare provider.
How should I store Palladone™?
- Store Palladone at room temperature, 59° to 86° F (15° to 30° C).
- Always keep Palladone in a safe place to protect from theft.
- Flush unused or out-of-date Palladone down the toilet.
- Keep Palladone™ out of the reach of children. Accidental use in children is a medical emergency and can result in death. If a child accidentally takes Palladone, call your local Poison Control Center or go to the nearest emergency room right away.
General informationabout the safe and effective use of Palladone
Use Palladone™ only for the pain for which it was prescribed. Do not give Palladone™ to other people, even if they have the same symptoms you have. Palladone can harm other people and even cause death. Sharing Palladone is against the law.
This Medication Guide summarizes the most important information about Palladone™. If you would like more information, talk with your healthcare provider. You can ask your pharmacist or other healthcare provider for information about Palladone™ that is written for health professionals or call Purdue Pharma at 1 (888)726-7535.
What are the ingredients of Palladone™?
Active Ingredient: hydromorphone hydrochloride
Inactive Ingredients: Pellets - ammonio methacrylate copolymer type B, ethylcellulose, and stearyl alcohol
Capsules - FD&C blue #2 (24 mg strength capsule only), gelatin, red iron oxide (12 mg and 16 mg strength capsules only), synthetic black iron oxide, and titanium dioxide
This Medication Guide has been approved by the U.S. Food and Drug Administration.
| PALLADONE
hydromorphone hydrochloride capsule, extended release |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| PALLADONE
hydromorphone hydrochloride capsule, extended release |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| PALLADONE
hydromorphone hydrochloride capsule, extended release |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| PALLADONE
hydromorphone hydrochloride capsule, extended release |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Purdue Pharma LP (932323652) |



