Label: QUINAPRIL tablet
-
Contains inactivated NDC Code(s)
NDC Code(s): 55111-621-10, 55111-621-90, 55111-622-10, 55111-622-90, view more55111-623-10, 55111-623-90, 55111-624-10, 55111-624-90 - Packager: Dr.Reddy's Laboratories Limited
- This is a repackaged label.
- Source NDC Code(s): 67787-267, 67787-268, 67787-269, 67787-270
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated September 9, 2009
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
BOXED WARNING
(What is this?)
USE IN PREGNANCY
When used in pregnancy during the second and third trimesters, ACE inhibitors can cause injury and even death to the developing fetus. When pregnancy is detected, quinapril tablets should be discontinued as soon as possible. See WARNINGS, Fetal/Neonatal Morbidity and Mortality
-
DESCRIPTION
Quinapril hydrochloride USP is the hydrochloride salt of quinapril, the ethyl ester of a non-sulfhydryl,angiotensin-converting enzyme (ACE) inhibitor, quinaprilat.
Quinapril hydrochloride USP is chemically described as [3S-[2[R*(R*)], 3R*]]-2-[2-[[1(ethoxycarbonyl)-3-phenylpropyl]amino]-1-oxopropyl]-1 ,2,3,4-tetrahydro-3-isoquinolinecarboxylicacid, monohydrochloride. Its empirical formula is C2SH30N20s -HCI and its structural formula is:
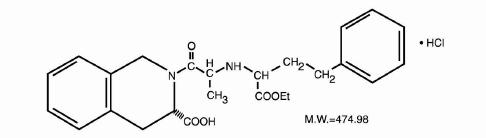
Quinapril hydrochloride USP is a white to off-white amorphous powder that is freely soluble inaqueous solvents.
Quinapril Tablets USP contain quinapril hydrochloride equivalent to 5 mg, 10 mg, 20 mg, or 40 mgof quinapril for oral administration.
Each tablet also contains lactose monohydrate, magnesium carbonate, magnesium stearate,crospovidone, povidone and opadry brown (hypromellose, titanium dioxide, ironoxide and macrogol).
-
CLINICAL PHARMACOLOGY
Mechanism of Action
Quinapril is deesterified to the principal metabolite, quinaprilat, which is aninhibitor of ACE activity in human subjects and animals. ACE is a peptidyl dipeptidase that catalyzesthe conversion of angiotensin I to the vasoconstrictor, angiotensin II. The effect of quinapril inhypertension appears to result primarily from the inhibition of circulating and tissue ACE activity,thereby reducing angiotensin II formation. Quinapril inhibits the elevation in blood pressure causedby intravenously administered angiotensin I, but has no effect on the pressor response toangiotensin II, norepinephrine or epinephrine. Angiotensin II also stimulates the secretion ofaldosterone from the adrenal cortex, thereby facilitating renal sodium and fluid reabsorption.Reduced aldosterone secretion by quinapril may result in a small increase in serum potassium. Incontrolled hypertension trials, treatment with quinapril hydrochloride alone resulted in meanincreases in potassium of 0.07 mmol/L (see PRECAUTIONS). Removal of angiotensin II negativefeedback on renin secretion leads to increased plasma renin activity (PRA).
While the principal mechanism of antihypertensive effect is thought to be through the reninangiotensin-aldosterone system, quinapril exerts antihypertensive actions even in patients with lowrenin hypertension. Quinapril hydrochloride was an effective antihypertensive in all races studied,although it was somewhat less effective in blacks (usually a predominantly low renin group) than innonblacks. ACE is identical to kininase II, an enzyme that degrades bradykinin, a potent peptidevasodilator; whether increased levels of bradykinin playa role in the therapeutic effect of quinaprilremains to be elucidated.
Pharmacokinetics and Metabolism
Following oral administration, peak plasma quinaprilconcentrations are observed within one hour. Based on recovery of quinapril and its metabolites inurine, the extent of absorption is at least 60%. The rate and extent of quinapril absorption arediminished moderately (approximately 25 to 30%) when quinapril hydrochloride tablets areadministered during a high-fat meal. Following absorption, quinapril is deesterified to its major activemetabolite, quinaprilat (about 38% of oral dose), and to other minor inactive metabolites. Followingmultiple oral dosing of quinapril hydrochloride, there is an effective accumulation half-life ofquinaprilat of approximately 3 hours, and peak plasma quinaprilat concentrations are observedapproximately 2 hours post-dose. Quinaprilat is eliminated primarily by renal excretion, up to 96% ofan IV dose, and has an elimination half-life in plasma of approximately 2 hours and a prolongedterminal phase with a half-life of 25 hours. The pharmacokinetics of quinapril and quinaprilat arelinear over a single-dose range of 5 to 80 mg doses and 40 to 160 mg in multiple daily doses.Approximately 97% of either quinapril or quinaprilat circulating in plasma is bound to proteins.
In patients with renal insufficiency, the elimination half-life of quinaprilat increases as creatinineclearance decreases. There is a linear correlation between plasma quinaprilat clearance andcreatinine clearance. In patients with end-stage renal disease, chronic hemodialysis or continuousambulatory peritoneal dialysis has little effect on the elimination of quinapril and quinaprilat.Elimination of quinaprilat may be reduced in elderly patients (≥65 years) and in those with heartfailure; this reduction is attributable to decrease in renal function (see DOSAGE ANDADMINISTRATION). Quinaprilat concentrations are reduced in patients with alcoholic cirrhosis dueto impaired deesterification of quinapril. Studies in rats indicate that quinapril and its metabolites donot cross the blood-brain barrier.
Pharmacodynamics and Clinical Effects
Hypertension
Single doses of 20 mg of quinapril hydrochloride provide over 80% inhibition ofplasma ACE for 24 hours. Inhibition of the pressor response to angiotensin I is shorter-lived, with a20 mg dose giving 75% inhibition for about 4 hours, 50% inhibition for about 8 hours, and 20%inhibition at 24 hours. With chronic dosing, however, there is substantial inhibition of angiotensin IIlevels at 24 hours by doses of 20 to 80 mg.
Administration of 10 to 80 mg of quinapril hydrochloride to patients with mild to severe hypertensionresults in a reduction of sitting and standing blood pressure to about the same extent with minimaleffect on heart rate. Symptomatic postural hypotension is infrequent although it can occur in patientswho are salt-and/or volume-depleted (see WARNINGS). Antihypertensive activity commences within1 hour with peak effects usually achieved by 2 to 4 hours after dosing. During chronic therapy, mostof the blood pressure lowering effect of a given dose is obtained in 1 to 2 weeks. In multiple-dosestudies, 10 to 80 mg per day in single or divided doses lowered systolic and diastolic blood pressurethroughout the dosing interval, with a trough effect of about 5 to 11/3 to 7 mm Hg. The trough effectrepresents about 50% of the peak effect. While the dose-response relationship is relatively flat,doses of 40 to 80 mg were somewhat more effective at trough than 10 to 20 mg, and twice dailydosing tended to give a somewhat lower trough blood pressure than once daily dosing with the sametotal dose. The antihypertensive effect of quinapril hydrochloride continues during long-term therapy,with no evidence of loss of effectiveness.
Hemodynamic assessments in patients with hypertension indicate that blood pressure reductionproduced by quinapril is accompanied by a reduction in total peripheral resistance and renalvascular resistance with little or no change in heart rate, cardiac index, renal blood flow, glomerularfiltration rate, or filtration fraction.
Use of quinapril hydrochloride with a thiazide diuretic gives a blood pressure lowering effect greaterthan that seen with either agent alone.
In patients with hypertension, quinapril hydrochloride 10 to 40 mg was similar in effectiveness tocaptopril, enalapril, propranolol, and thiazide diuretics.
Therapeutic effects appear to be the same for elderly (≥65 years of age) and younger adult patientsgiven the same daily dosages, with no increase in adverse events in elderly patients.
-
INDICATIONS AND USAGE
Hypertension
Quinapril hydrochloride is indicated for the treatment of hypertension. It may be used alone or incombination with thiazide diuretics.
In using quinapril hydrochloride, consideration should be given to the fact that another angiotensinconvertingenzyme inhibitor, captopril, has caused agranulocytosis, particularly in patients with renalimpairment or collagen vascular disease. Available data are insufficient to show that quinaprilhydrochloride does not have a similar risk (see WARNINGS).
Angioedema in black patients
Black patients receiving ACE inhibitor monotherapy have beenreported to have a higher incidence of angioedema compared to non-blacks. It should also be notedthat in controlled clinical trials ACE inhibitors have an effect on blood pressure that is less in blackpatients than in non-blacks.
- CONTRAINDICATIONS
-
WARNINGS
Anaphylactoid and Possibly Related Reactions
Presumably because angiotensin-converting inhibitors affect the metabolism of eicosanoids andpolypeptides, including endogenous bradykinin, patients receiving ACE inhibitors (including quinapril hydrochloride) may be subject to a variety of adverse reactions, some of them serious.
Head and Neck Angioedema
Angioedema of the face, extremities, lips, tongue, glottis, and larynxhas been reported in patients treated with ACE inhibitors and has been seen in 0.1% of patientsreceiving quinapril hydrochloride.
In two similarly sized U.S. postmarketing trials that, combined, enrolled over 3,000 black patients andover 19,000 non-blacks, angioedema was reported in 0.30% and 0.55% of blacks (in study 1 and 2respectively) and 0.39% and 0.17% of non-blacks.
Angioedema associated with laryngeal edema can be fatal. If laryngeal stridor or angioedema of theface, tongue, or glottis occurs, treatment with quinapril hydrochloride should be discontinuedimmediately, the patient treated in accordance with accepted medical care, and carefully observeduntil the swelling disappears. In instances where swelling is confined to the face and lips, thecondition generally resolves without treatment; antihistamines may be useful in relieving symptoms.Where there is involvement of the tongue, glottis, or larynx likely to cause airway obstruction,emergency therapy including, but not limited to, subcutaneous epinephrine solution 1:1000(0.3 to 0.5 mL) should be promptly administered (see ADVERSE REACTIONS).
Intestinal Angioedema
Intestinal angioedema has been reported in patients treated with ACEinhibitors. These patients presented with abdominal pain (with or without nausea or vomiting); insome cases there was no prior history of facial angioedema and C-1 esterase levels were normal.The angioedema was diagnosed by procedures including abdominal CT scan or ultrasound, or atsurgery, and symptoms resolved after stopping the ACE inhibitor. Intestinal angioedema should beincluded in the differential diagnosis of patients on ACE inhibitors presenting with abdominal pain.
Patients with a history of angioedema
Patients with a history of angioedema unrelated to ACEinhibitor therapy may be at increased risk of angioedema while receiving an ACE inhibitor (see also CONTRAINDICATIONS).
Anaphylactoid reactions during desensitization
Two patients undergoing desensitizingtreatment with hymenoptera venom while receiving ACE inhibitors sustained life-threateninganaphylactoid reactions. In the same patients, these reactions were avoided when ACE inhibitorswere temporarily withheld, but they reappeared upon inadvertent rechallenge.
Anaphylactoid reactions during membrane exposure
Anaphylactoid reactions have beenreported in patients dialyzed with high-flux membranes and treated concomitantly with an ACEinhibitor. Anaphylactoid reactions have also been reported in patients undergoing low-densitylipoprotein apheresis with dextran sulfate absorption.
Hepatic Failure
Rarely, ACE inhibitors have been associated with a syndrome that starts withcholestatic jaundice and progresses to fulminant hepatic necrosis and (sometimes) death. Themechanism of this syndrome is not understood. Patients receiving ACE inhibitors who developjaundice or marked elevations of hepatic enzymes should discontinue the ACE inhibitor and receiveappropriate medical follow-up.
Hypotension
Excessive hypotension is rare in patients with uncomplicated hypertension treatedwith quinapril hydrochloride alone. Patients with heart failure given quinapril hydrochloride commonlyhave some reduction in blood pressure, but discontinuation of therapy because of continuingsymptomatic hypotension usually is not necessary when dosing instructions are followed. Cautionshould be observed when initiating therapy in patients with heart failure (see DOSAGE ANDADMINISTRATION). In controlled studies, syncope was observed in 0.4% of patients (N=3203); thisincidence was similar to that observed for captopril (1 %) and enalapril (0.8%).
Patients at risk of excessive hypotension, sometimes associated with oliguria and/or progressiveazotemia, and rarely with acute renal failure and/or death, include patients with the followingconditions or characteristics: heart failure, hyponatremia, high dose diuretic therapy, recent intensivediuresis or increase in diuretic dose, renal dialysis, or severe volume and/or salt depletion of anyetiology. It may be advisable to eliminate the diuretic (except in patients with heart failure), reducethe diuretic dose or cautiously increase salt intake (except in patients with heart failure) beforeinitiating therapy with quinapril hydrochloride in patients at risk for excessive hypotension who areable to tolerate such adjustments.
In patients at risk of excessive hypotension, therapy with quinapril hydrochloride should be startedunder close medical supervision. Such patients should be followed closely for the first two weeks oftreatment and whenever the dose of quinapril hydrochloride and/or diuretic is increased. Similarconsiderations may apply to patients with ischemic heart or cerebrovascular disease in whom anexcessive fall in blood pressure could result in a myocardial infarction or a cerebrovascular accident.
If excessive hypotension occurs, the patient should be placed in the supine position and, ifnecessary, receive an intravenous infusion of normal saline. A transient hypotensive response is nota contraindication to further doses of quinapril hydrochloride, which usually can be given withoutdifficulty once the blood pressure has stabilized. If symptomatic hypotension develops, a dosereduction or discontinuation of quinapril hydrochloride or concomitant diuretic may be necessary.
Neutropenia/Agranulocytosis
Another ACE inhibitor, captopril, has been shown to causeagranulocytosis and bone marrow depression rarely in patients with uncomplicated hypertension,but more frequently in patients with renal impairment, especially if they also have a collagen vasculardisease, such as systemic lupus erythematosus or scleroderma. Agranulocytosis did occur duringquinapril hydrochloride treatment in one patient with a history of neutropenia during previouscaptopril therapy. Available data from clinical trials of quinapril hydrochloride are insufficient to showthat, in patients without prior reactions to other ACE inhibitors, quinapril hydrochloride does notcause agranulocytosis at similar rates. As with other ACE inhibitors, periodic monitoring of whiteblood cell counts in patients with collagen vascular disease and/or renal disease should beconsidered.
Fetal/Neonatal Morbidity and Mortality
ACE inhibitors can cause fetal and neonatal morbidity anddeath when administered to pregnant women. Several dozen cases have been reported in the worldliterature. When pregnancy is detected, ACE inhibitors should be discontinued as soon as possible.
The use of ACE inhibitors during the second and third trimesters of pregnancy has been associatedwith fetal and neonatal injury, including hypotension, neonatal skull hypoplasia, anuria, reversible orirreversible renal failure, and death. Oligohydramnios has also been reported, presumably resultingfrom decreased fetal renal function; oligohydramnios in this setting has been associated with fetallimb contractures, craniofacial deformation, and hypoplastic lung development. Prematurity,intrauterine growth retardation, and patent ductus arteriosus have also been reported, although it isnot clear whether these occurrences were due to the ACE inhibitor exposure.
These adverse effects do not appear to have resulted from intrauterine ACE inhibitor exposure thathas been limited to the first trimester. Mothers whose embryos and fetuses are exposed to ACEinhibitors only during the first trimester should be so informed. Nonetheless, when patients becomepregnant, physicians should make every effort to discontinue the use of quinapril hydrochloride assoon as possible.
Rarely (probably less often than once in every thousand pregnancies), no alternative to ACEinhibitors will be found. In these rare cases, the mothers should be apprised of the potential hazardsto their fetuses, and serial ultrasound examinations should be performed to assess the intraamnioticenvironment.
If oligohydramnios is observed, quinapril hydrochloride should be discontinued unless it isconsidered lifesaving for the mother. Contraction stress testing (CST), a non-stress test (NST), orbiophysical profiling (BPP) may be appropriate, depending upon the week of pregnancy. Patientsand physicians should be aware, however, that oligohydramnios may not appear until after the fetushas sustained irreversible injury.
Infants with histories of in utero exposure to ACE inhibitors should be closely observed forhypotension, oliguria, and hyperkalemia. If oliguria occurs, attention should be directed towardsupport of blood pressure and renal perfusion. Exchange transfusion or dialysis may be required asa means of reversing hypotension and/or substituting for disordered renal function. Removal ofquinapril hydrochloride, which crosses the placenta, from the neonatal circulation is not significantlyaccelerated by these means.
No teratogenic effects of quinapril hydrochloride were seen in studies of pregnant rats and rabbits.On a mg/kg basis, the doses used were up to 180 times (in rats) and one time (in rabbits) themaximum recommended human dose.
-
PRECAUTIONS
General
Impaired renal function
As a consequence of inhibiting the renin-angiotensinaldosterone system,changes in renal function may be anticipated in susceptible individuals. In patients with severe heartfailure whose renal function may depend on the activity of the renin-angiotensinaldosterone system,treatment with ACE inhibitors, including quinapril hydrochloride, may be associated with oliguriaand/or progressive azotemia and rarely acute renal failure and/or death.
In clinical studies in hypertensive patients with unilateral or bilateral renal artery stenosis, increasesin blood urea nitrogen and serum creatinine have been observed in some patients following ACEinhibitor therapy. These increases were almost always reversible upon discontinuation of the ACEinhibitor and/or diuretic therapy. In such patients, renal function should be monitored during the firstfew weeks of therapy.
Some patients with hypertension or heart failure with no apparent preexisting renal vascular diseasehave developed increases in blood urea and serum creatinine, usually minor and transient,especially when quinapril hydrochloride has been given concomitantly with a diuretic. This is morelikely to occur in patients with preexisting renal impairment. Dosage reduction and/or discontinuationof any diuretic and/or quinapril hydrochloride may be required.
Evaluation of patients with hypertension or heart failure should always include assessmentof renal function (see DOSAGE AND ADMINISTRATION).
Hyperkalemia and potassium-sparing diuretics: In clinical trials, hyperkalemia (serum potassium≥5.8 mmol/L) occurred in approximately 2% of patients receiving quinapril hydrochloride. In mostcases, elevated serum potassium levels were isolated values which resolved despite continuedtherapy. Less than 0.1% of patients discontinued therapy due to hyperkalemia. Risk factors for thedevelopment of hyperkalemia include renal insufficiency, diabetes mellitus, and the concomitant useof potassium-sparing diuretics, potassium supplements, and/or potassium containing saltsubstitutes, which should be used cautiously, if at all, with quinapril hydrochloride (see PRECAUTIONS, Drug Interactions).
Cough
Presumably due to the inhibition of the degradation of endogenous bradykinin, persistentnon-productive cough has been reported with all ACE inhibitors, always resolving afterdiscontinuation of therapy. ACE inhibitor-induced cough should be considered in the differentialdiagnosis of cough.
Surgery/anesthesia
In patients undergoing major surgery or during anesthesia with agents thatproduce hypotension, quinapril hydrochloride will block angiotensin II formation secondary tocompensatory renin release. If hypotension occurs and is considered to be due to this mechanism,it can be corrected by volume expansion.
Information for Patients
Pregnancy
Female patients of childbearing age should be told about the consequences of secondandthird-trimester exposure to ACE inhibitors, and they should also be told that theseconsequences do not appear to have resulted from intrauterine ACE-inhibitor exposure that hasbeen limited to the first trimester. These patients should be asked to report pregnancies to theirphysicians as soon as possible.
Angioedema
Angioedema, including laryngeal edema can occur with treatment with ACEinhibitors, especially following the first dose. Patients should be so advised and told to reportimmediately any signs or symptoms suggesting angioedema (swelling of face extremities, eyes, lips,tongue, difficulty in swallowing or breathing: and to stop taking the drug until they have consultedwith their physician (see WARNINGS).
Symptomatic hypotension
Patients should be cautioned that light-headedness can occur,especially during the first few days of quinapril hydrochloride therapy, and that it should be reportedto a physician. If actual syncope occurs, patients should be told to not take the drug until they haveconsulted with their physician (see WARNINGS).
All patients should be cautioned that inadequate fluid intake or excessive perspiration, diarrhea, orvomiting can lead to an excessive fall in blood pressure because of reduction in fluid volume, withthe same consequences of lightheadedness and possible syncope.
Patients planning to undergo any surgery and/or anesthesia should be told to inform their physicianthat they are taking an ACE inhibitor.
Hyperkalemia
Patients should be told not to use potassium supplements or salt substitutescontaining potassium without consulting their physician (see PRECAUTIONS).
Neutropenia
Patients should be told to report promptly any indication of infection (eg, sore throat,fever) which could be a sign of neutropenia.
NOTE: As with many other drugs, certain advice to patients being treated with quinaprilhydrochloride is warranted. This information is intended to aid in the safe and effective use of thismedication. It is not a disclosure of all possible adverse or intended effects.
Drug Interactions
Concomitant diuretic therapy
As with other ACE inhibitors, patients on diuretics, especially thoseon recently instituted diuretic therapy may occasionally experience an excessive reduction of bloodpressure after initiation of therapy with quinapril hydrochloride. The possibility of hypotensive effectswith quinapril hydrochloride may be minimized by either discontinuing the diuretic or cautiouslyincreasing salt intake prior to initiation of treatment with quinapril hydrochloride. If it is not possibleto discontinue the diuretic, the starting dose of quinapril should be reduced (see DOSAGE ANDADMINISTRATION).
Agents increasing serum potassium
Quinapril can attenuate potassium loss caused by thiazidediuretics and increase serum potassium when used alone. If concomitant therapy of quinaprilhydrochloride with potassium-sparing diuretics (eg, spironolactone, triamterene, or amiloride),potassium supplements, or potassium-containing salt substitutes is indicated, they should be usedwith caution along with appropriate monitoring of serum potassium (see PRECAUTIONS).
Tetracycline and other drugs that interact with magnesium
Simultaneous administration oftetracycline with quinapril hydrochloride reduced the absorption of tetracycline by approximately28% to 37%, possibly due to the high magnesium content in quinapril hydrochloride tablets. Thisinteraction should be considered if coprescribing quinapril hydrochloride and tetracycline or otherdrugs that interact with magnesium.
Lithium
Increased serum lithium levels and symptoms of lithium toxicity have been reported inpatients receiving concomitant lithium and ACE inhibitor therapy. These drugs should becoadministered with caution and frequent monitoring of serum lithium levels is recommended. If adiuretic is also used, it may increase the risk of lithium toxicity.
Other agents
Drug interaction studies of quinapril hydrochloride with other agents showed:
-
Multiple dose therapy with propranolol or cimetidine has no effect on the pharmacokinetics ofsingle doses of quinapril hydrochloride.
-
The anticoagulant effect of a single dose of warfarin (measured by prothrombin time) was notsignificantly changed by quinapril coadministration twice-daily.
-
quinapril hydrochloride treatment did not affect the pharmacokinetics of digoxin.
-
No pharmacokinetic interaction was observed when single doses of quinapril hydrochloride andhydrochlorothiazide were administered concomitantly.
-
Co-administration of multiple 10 mg doses of atorvastatin with 80 mg of quinapril hydrochlorideresulted in no significant change in the steady-state pharmacokinetic parameters of atorvastatin.
Carcinogenesis, Mutagenesis, Impairment of Fertility
Quinapril hydrochloride was not carcinogenic in mice or rats when given in doses up to 75 or100 mg/kg/day (50 to 60 times the maximum human daily dose, respectively, on an mg/kg basis and3.8 to 10 times the maximum human daily dose when based on an mg/m2 basis) for 104 weeks.Female rats given the highest dose level had an increased incidence of mesenteric lymph nodehemangiomas and skin/subcutaneous lipomas. Neither quinapril nor quinaprilat were mutagenic inthe Ames bacterial assay with or without metabolic activation. Quinapril was also negative in thefollowing genetic toxicology studies: in vitro mammalian cell point mutation, sister chromatidexchange in cultured mammalian cells, micronucleus test with mice, in vitro chromosome aberrationwith V79 cultured lung cells, and in an in vivo cytogenetic study with rat bone marrow. There wereno adverse effects on fertility or reproduction in rats at doses up to 100 mg/kg/day (60 and 10 timesthe maximum daily human dose when based on mg/kg and mg/m2 , respectively).
Pregnancy: Teratogenic Effects: Pregnancy: Category B.
Pregnancy Categories C (first trimester) and D (second and third trimesters): See WARNINGS,Fetal/Neonatal Morbidity and Mortality.
Nursing Mothers
Because quinapril hydrochloride is secreted in human milk, caution should be exercised when thisdrug is administered to a nursing woman.
Pediatric Use
The safety and effectiveness of quinapril hydrochloride in pediatric patients have not beenestablished.
Geriatric Use
Clinical studies of quinapril hydrochloride did not include sufficient numbers of subjects aged 65 andover to determine whether they respond differently from younger subjects. Other reported clinicalexperience has not identified differences in responses between the elderly and younger patients.
In general, dose selection for an elderly patient should be cautious, usually starting at the low endof the dosing range, reflecting the greater frequency of decreased hepatic, renal or cardiac function,and of concomitant disease or other drug therapy.
This drug is known to be substantially excreted by the kidney, and the risk of toxic reactions to thisdrug may be greater in patients with impaired renal function. Because elderly patients are more likelyto have decreased renal function, care should be taken in dose selection, and it may be useful tomonitor renal function.
Elderly patients exhibited increased area under the plasma concentration time curve and peak levelsfor quinaprilat compared to values observed in younger patients; this appeared to relate todecreased renal function rather than to age itself.
-
-
ADVERSE REACTIONS
Hypertension
Quinapril hydrochloride has been evaluated for safety in 4960 subjects and patients. Of these, 3203patients, including 655 elderly patients, participated in controlled clinical trials. Quinaprilhydrochloride has been evaluated for long-term safety in over 1400 patients treated for 1 year ormore.
Adverse experiences were usually mild and transient.
In placebo-controlled trials, discontinuation of therapy because of adverse events was required in4.7% of patients with hypertension.
Adverse experiences probably or possibly related to therapy or of unknown relationship to therapyoccurring in 1% or more of the 1563 patients in placebo-controlled hypertension trials who weretreated with quinapril hydrochloride are shown below.
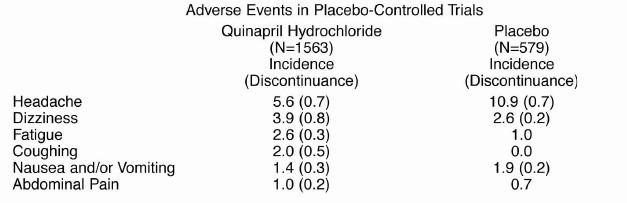
Hypertension
Clinical adverse experiences probably, possibly, or definitely related, or of uncertain relationship totherapy occurring in 0.5% to 1.0% (except as noted) of the patients with hypertension treated withquinapril hydrochloride (With or without concomitant diuretic) in controlled or uncontrolled trials(N=4847) and less frequent, clinically significant events seen in clinical trials or postmarketingexperience (the rarer events are in italics) include (listed by body system):
Cardiovascular
palpitation, vasodilation, tachycardia, heart failure, hyperkalemia, myocardial infarction, cerebrovascular accident, hypertensive crisis, angina pectoris, orthostatic hypotension,cardiac rhythm disturbances, cardiogenic shock
Gastrointestinal
flatulence, dry mouth or throat, constipation, gastrointestinal hemorrhage, pancreatitis, abnormal liver function tests, dyspepsia
Integumentary
alopecia, increased sweating, pemphigus, pruritus, exfoliative dermatitis, photosensitivity reaction, dermatopolymyositis
Angioedema
Angioedema has been reported in patients receiving quinapril hydrochloride (0.1 %). Angioedemaassociated with laryngeal edema may be fatal. If angioedema of the face, extremities, lips, tongue,glottis, and/or larynx occurs, treatment with quinapril hydrochloride should be discontinued andappropriate therapy instituted immediately. (See WARNINGS.)
Creatinine and Blood Urea Nitrogen
Increases (>1.25 times the upper limit of normal) in serumcreatinine and blood urea nitrogen were observed in 2% and 2%, respectively, of all patients treatedwith quinapril hydrochloride alone. Increases are more likely to occur in patients receivingconcomitant diuretic therapy than in those on quinapril hydrochloride alone. These increases oftenremit on continued therapy.
-
OVERDOSAGE
Doses of 1440 to 4280 mg/kg of quinapril cause significant lethality in mice and rats. No specificinformation is available on the treatment of overdosage with quinapril. The most likely clinicalmanifestation would be symptoms attributable to severe hypotension.
Laboratory determinations of serum levels of quinapril and its metabolites are not widely available,and such determinations have, in any event, no established role in the management of quinapriloverdose.
No data are available to suggest physiological maneuvers (eg, maneuvers to change pH of the urine)that might accelerate elimination of quinapril and its metabolites.
Hemodialysis and peritoneal dialysis have little effect on the elimination of quinapril and quinaprilat.Angiotensin II could presumably serve as a specific antagonist-antidote in the setting of quinapriloverdose, but angiotensin II is essentially unavailable outside of scattered research facilities.Because the hypotensive effect of quinapril is achieved through vasodilation and effectivehypovolemia, it is reasonable to treat quinapril overdose by infusion of normal saline solution.
-
DOSAGE AND ADMINISTRATION
Hypertension
Monotherapy
The recommended initial dosage of quinapril hydrochloride in patients not ondiuretics is 10 or 20 mg once daily. Dosage should be adjusted according to blood pressureresponse measured at peak (2 to 6 hours after dosing) and trough (predosing). Generally, dosageadjustments should be made at intervals of at least 2 weeks. Most patients have required dosagesof 20, 40, or 80 mg/day, given as a single dose or in two equally divided doses. In some patientstreated once daily, the antihypertensive effect may diminish toward the end of the dosing interval. Insuch patients an increase in dosage or twice daily administration may be warranted. In general,doses of 40 to 80 mg and divided doses give a somewhat greater effect at the end of the dosinginterval.
Concomitant Diuretics
If blood pressure is not adequately controlled with quinapril hydrochloridemonotherapy, a diuretic may be added. In patients who are currently being treated with a diuretic,symptomatic hypotension occasionally can occur following the initial dose of quinapril hydrochloride.To reduce the likelihood of hypotension, the diuretic should, if possible, be discontinued 2 to 3 daysprior to beginning therapy with quinapril hydrochloride (see WARNINGS). Then, if blood pressure isnot controlled with quinapril hydrochloride alone, diuretic therapy should be resumed.
If the diuretic cannot be discontinued, an initial dose of 5 mg quinapril hydrochloride should be usedwith careful medical supervision for several hours and until blood pressure has stabilized.
The dosage should subsequently be titrated (as described above) to the optimal response (seeWARNINGS, PRECAUTIONS, and Drug Interactions).
Renal Impairment
Kinetic data indicate that the apparent elimination half-life of quinaprilatincreases as creatinine clearance decreases. Recommended starting doses, based on clinical andpharmacokinetic data from patients with renal impairment, are as follows:
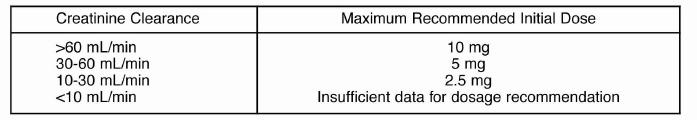
Patients should subsequently have their dosage titrated (as described above) to the optimalresponse.
Elderly (≥65 years)
The recommended initial dosage of quinapril hydrochloride in elderly patientsis 10 mg given once daily followed by titration (as described above) to the optimal response.
Following the initial dose of quinapril hydrochloride, the patient should be observed under medicalsupervision for at least two hours for the presence of hypotension or orthostatis and, if present, untilblood pressure stabilizes. The appearance of hypotension, orthostatis, or azotemia early in dosetitration should not preclude further careful dose titration. Consideration should be given to reducingthe dose of concomitant diuretics.
DOSE ADJUSTMENTS IN PATIENTS WITH HEART FAILURE AND RENAL IMPAIRMENT OR HYPONATREMIA
Pharmacokinetic data indicate that quinapril elimination is dependent on level of renal function. Inpatients with heart failure and renal impairment, the recommended initial dose of quinaprilhydrochloride is 5 mg in patients with a creatinine clearance above 30 mLlmin and 2.5 mg in patientswith a creatinine clearance of 10 to 30 mL/min. There is insufficient data for dosage recommendationin patients with a creatinine clearance less than 10 mLlmin (see DOSAGE AND ADMINISTRATION,Heart Failure,WARNINGS, and PRECAUTIONS, Drug Interactions).
If the initial dose is well tolerated, quinapril hydrochloride may be administered the following day asa twice daily regimen. In the absence of excessive hypotension or significant deterioration of renalfunction, the dose may be increased at weekly intervals based on clinical and hemodynamicresponse.
-
HOW SUPPLIED
Quinapril Tablets USP are supplied as follows:
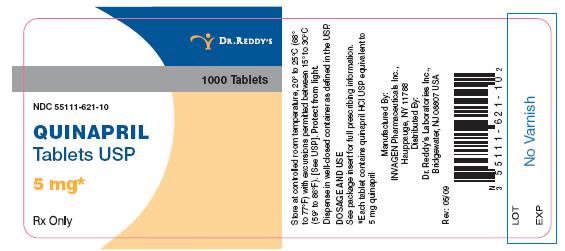
5-mg tablets: brown, round biconvex tablets de-bossed with I on the left side of bisect and G on theright side of bisect and 267 on other.
NDC 55111-621-90 bottles of 90 tablets
NDC 55111-621 -1 a bottles of 1000 tablets
10-mg tablets: brown, round biconvex tablets de-bossed with IG on one side and 268 on other.
NDC 55111-622-90 bottles of 90 tablets
NDC 55111-622-1 a bottles of 1000 tablets
20-mg tablets: brown, round biconvex tablets de-bossed with IG on one side and 269 on other.
NDC 55111-623-90 bottles of 90 tablets
NDC 55111-623-1 a bottles of 1000 tablets
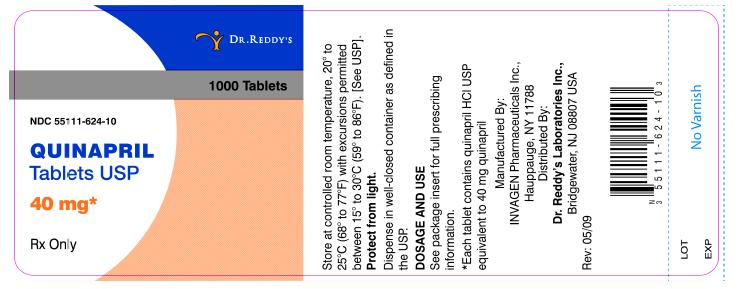
40-mg tablets: brown, oval biconvex tablets de-bossed with IG on one side and 270 on other.
NDC 55111-624-90 bottles of 90 tablets
NDC 55111-624-10 bottles of 1000 tablets
Dispense in well-closed containers as defined in the USP.
Store at controlled room temperature, 20° to 25°C (68° to 7JOF) with excursions permitted betweenPlacebo 15° to 30°C (59° to 86°F). [See USP]. Protect from light.
Manufactured by:
InvaGen Pharmaceuticals, Inc
Hauppauge, NY 11788
Distributed by:
Dr. Reddy's Laboratories Inc.,
Bridgewater, NJ 08807 USA
Rev: 05/09
- Labeling
-
INGREDIENTS AND APPEARANCE
QUINAPRIL
quinapril tabletProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:55111-621(NDC:67787-267) Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength quinapril hydrochloride (UNII: 33067B3N2M) (quinapril - UNII:RJ84Y44811) quinapril 5 mg Inactive Ingredients Ingredient Name Strength lactose monohydrate (UNII: EWQ57Q8I5X) magnesium carbonate (UNII: 0E53J927NA) magnesium stearate (UNII: 70097M6I30) crospovidone (UNII: 68401960MK) polyethylene glycol (UNII: 3WJQ0SDW1A) ferric oxide red (UNII: 1K09F3G675) ferric oxide yellow (UNII: EX438O2MRT) povidone (UNII: FZ989GH94E) hypromellose (UNII: 3NXW29V3WO) titanium dioxide (UNII: 15FIX9V2JP) Product Characteristics Color BROWN Score 2 pieces Shape ROUND Size 6mm Flavor Imprint Code I;G;267 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:55111-621-90 90 in 1 BOTTLE 2 NDC:55111-621-10 1000 in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA078457 07/20/2007 QUINAPRIL
quinapril tabletProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:55111-622(NDC:67787-268) Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength quinapril hydrochloride (UNII: 33067B3N2M) (quinapril - UNII:RJ84Y44811) quinapril 10 mg Inactive Ingredients Ingredient Name Strength lactose monohydrate (UNII: EWQ57Q8I5X) magnesium carbonate (UNII: 0E53J927NA) magnesium stearate (UNII: 70097M6I30) crospovidone (UNII: 68401960MK) povidone (UNII: FZ989GH94E) hypromellose (UNII: 3NXW29V3WO) titanium dioxide (UNII: 15FIX9V2JP) polyethylene glycol (UNII: 3WJQ0SDW1A) ferric oxide red (UNII: 1K09F3G675) ferric oxide yellow (UNII: EX438O2MRT) Product Characteristics Color BROWN Score 2 pieces Shape ROUND Size 6mm Flavor Imprint Code IG;268 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:55111-622-90 90 in 1 BOTTLE 2 NDC:55111-622-10 1000 in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA078457 07/20/2007 QUINAPRIL
quinapril tabletProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:55111-623(NDC:67787-269) Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength quinapril hydrochloride (UNII: 33067B3N2M) (quinapril - UNII:RJ84Y44811) quinapril 20 mg Inactive Ingredients Ingredient Name Strength lactose monohydrate (UNII: EWQ57Q8I5X) magnesium carbonate (UNII: 0E53J927NA) magnesium stearate (UNII: 70097M6I30) crospovidone (UNII: 68401960MK) povidone (UNII: FZ989GH94E) hypromellose (UNII: 3NXW29V3WO) titanium dioxide (UNII: 15FIX9V2JP) polyethylene glycol (UNII: 3WJQ0SDW1A) ferric oxide red (UNII: 1K09F3G675) ferric oxide yellow (UNII: EX438O2MRT) Product Characteristics Color BROWN Score 2 pieces Shape ROUND Size 7mm Flavor Imprint Code IG;269 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:55111-623-90 90 in 1 BOTTLE 2 NDC:55111-623-10 1000 in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA078457 07/20/2007 QUINAPRIL
quinapril tabletProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:55111-624(NDC:67787-270) Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength quinapril hydrochloride (UNII: 33067B3N2M) (quinapril - UNII:RJ84Y44811) quinapril 40 mg Inactive Ingredients Ingredient Name Strength lactose monohydrate (UNII: EWQ57Q8I5X) magnesium carbonate (UNII: 0E53J927NA) magnesium stearate (UNII: 70097M6I30) crospovidone (UNII: 68401960MK) povidone (UNII: FZ989GH94E) hypromellose (UNII: 3NXW29V3WO) titanium dioxide (UNII: 15FIX9V2JP) polyethylene glycol (UNII: 3WJQ0SDW1A) ferric oxide red (UNII: 1K09F3G675) ferric oxide yellow (UNII: EX438O2MRT) Product Characteristics Color BROWN Score 2 pieces Shape ROUND Size 6mm Flavor Imprint Code IG;270 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:55111-624-90 90 in 1 BOTTLE 2 NDC:55111-624-10 1000 in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA078457 07/20/2007 Labeler - Dr.Reddy's Laboratories Limited (862179079) Establishment Name Address ID/FEI Business Operations InvaGen Pharmaceuticals, Inc. 165104469 analysis, manufacture