ATROPINE SULFATE - atropine sulfate solution
Sandoz Inc.
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
----------
Atropine Sulfate Ophthalmic Solution, 1%
DESCRIPTION
Atropine sulfate ophthalmic solution, 1% is an anticholinergic prepared as a sterile topical ophthalmic solution. The active ingredient is represented by the chemical structure:
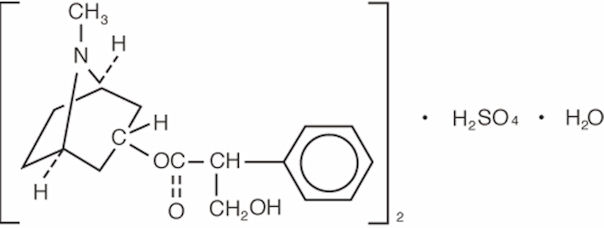
Established name: atropine sulfate
Chemical name: benzeneacetic acid, α-(hydroxymethyl)-, 8-methyl-8-aza-bicyclo-[3.2.1]oct-3-yl ester, endo-(±)-, sulfate (2:1) (salt), monohydrate.
Each mL contains: Active: atropine sulfate 1.0%. Preservative: ben zalkonium chloride 0.01%. Vehicle: 0.5% hypromellose 2910. Inactives: boric acid, sodium hydroxide and/or hydrochloric acid (to adjust pH), purified water.
CLINICAL PHARMACOLOGY
This anticholinergic preparation blocks the responses of the sphincter muscle of the iris and the accommodative muscle of the ciliary body to cholinergic stimulation, producing pupillary dilation (mydriasis) and paralysis of accommodation (cycloplegia).
INDICATIONS & USAGE
For mydriasis and/or cycloplegia. For cycloplegic refraction, for papillary dilation desired in inflammatory conditions of the iris and uveal tract.
CONTRAINDICATIONS
Contraindicated in persons with primary glaucoma or a tendency toward glaucoma, e.g., narrow anterior chamber angle, and in those persons showing hypersensitivity to any component of this preparation.
WARNINGS
FOR TOPICAL OPHTHALMIC USE ONLY – NOT FOR INJECTION. Excessive use in certain individuals with a previous history of susceptibility to belladonna alkaloids may produce systemic symptoms of atropine poisoning.
PRECAUTIONS
General
To avoid excessive systemic absorption, the lacrimal sac should be compressed by digital pressure for two to three minutes after instillation. To avoid inducing angle closure glaucoma, an estimation of the depth of the angle of the anterior chamber should be made. Administration of atropine in infants requires great caution.
Information for Patients
Do not touch dropper tip to any surface, as this may contaminate the solution.
Carcinogenesis, mutagenesis, impairment of fertility
No studies have been conducted in animals or in humans to evaluate the potential of these effects.
Pregnancy Category C
Animal reproduction studies have not been performed with atropine. It is also not known whether atropine can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. Atropine should be given to pregnant women only if clearly needed.
PATIENT WARNING
Patient should be advised not to drive or engage in other hazardous activities while pupils are dilated. Patient may experience sensitivity to light and should protect eyes in bright illumination during dilation. Parents should be warned not to get this preparation in their child’s mouth and to wash their hands following administration.
ADVERSE REACTIONS
Prolonged use may produce local irritation character ized by follicular conjunctivitis, vascular congestion, edema, exudate, and an eczematoid dermatitis. Severe reactions are manifested by hypotension with progressive respiratory depression. Coma and death have been reported in the very young.
OVERDOSAGE
Systemic atropine toxicity is manifested by flushing and dryness of the skin (a rash may be present in children), blurred vision, a rapid and irregular pulse, fever, abdominal distention in infants, mental aberration (hallucinosis) and loss of neuromuscular coordination. Atropine poisoning, although distressing, is rarely fatal even with large doses of atropine, and is self-limited if the cause is recognized and the atropine medication discontinued. Treatment includes supportive measures including maintaining a patent airway and assisting respiration if needed. Treat hyperthermia, coma and seizures if they occur (1). In infants and children, the body surface must be kept moist. Excitement may be controlled by diazepam or a short acting barbiturate. For ingestion, activated charcoal can be used to prevent drug absorption. If necessary, ipecac or another cathartic may be useful for drug removal during initial treatment (1,2). Physostigmine is used as an antidote to the systemic effects of atropine and may be administered parenterally to provide more prompt relief of intoxication. Parenteral physostigmine may be particularly useful in cases of pronounced hallucinations, agitation in which a patient may be dangerous to himself or others, arrhythmias resulting in uncontrolled hemodynamic instability, and intractable seizures.
DOSAGE & ADMINISTRATION
Adults: For uveitis, administer one or two drops topically to the eye(s) up to four times daily.
The lacrimal sac should be compressed by digital pressure for two to three minutes after instillation. Heavily pigmented irides may require larger doses.
HOW SUPPLIED
Supplied in 5 mL and 15 mL in plastic DROP-TAINER* dispensers.
5 mL: NDC 61314-303-01
15 mL: NDC 61314-303-02
REFERENCES
1. Kirk M, Kulig K, Rumack BH. Anticholinergics. In: Clinical Management of Poisoning and Drug Overdose, Second Edition. Edited by Haddad LM, Winchester JF. Philadelphia, W.B. Sauders Company, 1990, p 861-867.
2. Tani SA. Anticholinergics. In: Poisoning and Drug Overdose, Second Edition. Olson KR. Norwalk, CT, Appleton & Lange, 1994, p 75-76.
Revised: August 2003
*DROP-TAINER is a registered trademark of Alcon Manufacturing, Ltd.
340909-0803
Dist. by:
FALCON Pharmaceuticals, Ltd.
Fort Worth, Texas 76134 USA
Mfd. by:
ALCON LABORATORIES, INC.
Fort Worth, Texas 76134 USA
Printed in USA
| ATROPINE SULFATE
atropine sulfate solution |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Sandoz Inc. (005387188) |

