PYRIDOSTIGMINE BROMIDE- pyridostigmine bromide tablet
Sandoz Inc
----------
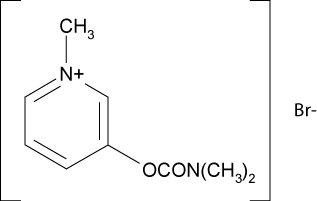
DESCRIPTION: Pyridostigmine bromide is an orally active cholinesterase inhibitor. Chemically, pyridostigmine bromide is 3-hydroxy-1-methylpyridinium bromide dimethylcarbamate. The molecular formula of pyridostigmine bromide is C9H13BrN2O2 and molecular weight is 261.12. Its structural formula is:
Each pyridostigmine bromide tablet intended for oral administration contains 60 mg of pyridostigmine bromide USP. In addition, it also contains the following inactive ingredients: colloidal silicon dioxide, anhydrous lactose and stearic acid.
CLINICAL PHARMACOLOGY
Pyridostigmine bromide inhibits the destruction of acetylcholine by cholinesterase and thereby permits freer transmission of nerve impulses across the neuromuscular junction. Pyridostigmine is an analog of neostigmine, but differs from it in certain clinically significant respects; for example, pyridostigmine is characterized by a longer duration of action and fewer gastrointestinal side effects.
CONTRAINDICATIONS
Pyridostigmine bromide is contraindicated in mechanical intestinal or urinary obstruction, and particular caution should be used in its administration to patients with bronchial asthma. Care should be observed in the use of atropine for counteracting side effects, as discussed below.
WARNINGS
Although failure of patients to show clinical improvement may reflect underdosage, it can also be indicative of overdosage. As is true of all cholinergic drugs, overdosage of pyridostigmine bromide may result in cholinergic crisis, a state characterized by increasing muscle weakness which, through involvement of the muscles of respiration, may lead to death. Myasthenic crisis due to an increase in the severity of the disease is also accompanied by extreme muscle weakness, and thus may be difficult to distinguish from cholinergic crisis on a symptomatic basis. Such differentiation is extremely important, since increases in doses of pyridostigmine bromide or other drugs of this class in the presence of cholinergic crisis or of a refractory or “insensitive” state could have grave consequences. Osserman and Genkins1 indicate that the differential diagnosis of the two types of crisis may require the use of edrophonium chloride as well as clinical judgment. The treatment of the two conditions obviously differs radically. Whereas the presence of myasthenic crisis suggests the need for more intensive anticholinesterase therapy, the diagnosis of cholinergic crisis, according to Osserman and Genkins1, calls for the prompt withdrawal of all drugs of this type. The immediate use of atropine in cholinergic crisis is also recommended.
Atropine may also be used to abolish or obtund gastrointestinal side effects or other muscarinic reactions; but such use, by masking signs of overdosage, can lead to inadvertent induction of cholinergic crisis.
For detailed information on the management of patients with myasthenia gravis, the physician is referred to one of the excellent reviews such as those by Osserman and Genkins,2 Grob3 or Schwab.4,5
PRECAUTIONS
Pyridostigmine is mainly excreted unchanged by the kidney.6,7,8 Therefore, lower doses may be required in patients with renal disease, and treatment should be based on titration of drug dosage to effect.6,7
ADVERSE REACTIONS
The side effects of pyridostigmine bromide are most commonly related to overdosage and generally are of two varieties, muscarinic and nicotinic. Among those in the former group are nausea, vomiting, diarrhea, abdominal cramps, increased peristalsis, increased salivation, increased bronchial secretions, miosis and diaphoresis. Nicotinic side effects are comprised chiefly of muscle cramps, fasciculation and weakness. Muscarinic side effects can usually be counteracted by atropine, but for reasons shown in the preceding section the expedient is not without danger. As with any compound containing the bromide radical, a skin rash may be seen in an occasional patient. Such reactions usually subside promptly upon discontinuance of the medication.
DOSAGE AND ADMINISTRATION: Pyridostigmine bromide is available as tablet form; each containing 60 mg pyridostigmine bromide.
Dosage: The size and frequency of the dosage must be adjusted to the needs of the individual patient.
The average dose is ten 60-mg tablets daily, spaced to provide maximum relief when maximum strength is needed. In severe cases as many as 25 tablets a day may be required, while in mild cases one to six tablets a day may suffice.
Note: For information on a diagnostic test for myasthenia gravis, and for the evaluation and stabilization of therapy, please see product literature on Tensilon® (edrophonium chloride).
HOW SUPPLIED Pyridostigmine bromide tablets 60 mg are supplied as white, round compressed tablets debossed “cor” over “128” on one side and scored (quadrisect) on the other side.
They are supplied as follows:
Bottles of 100 (NDC 0781-5015-01)
Bottles of 500 (NDC 0781-5015-05)
Store at controlled room temperature 15° - 30°C (59° - 86°F) (see USP). Dispense in tight containers as defined in USP/NF.
IMPORTANT: These tablets are hygroscopic. Keep in a dry place with the silica gel enclosed.
REFERENCES
- Osserman KE, Genkins G. Studies in myasthenia gravis: Reduction in mortality rate after crisis. JAMA. Jan 1963; 183:97-101.
- Osserman KE, Genkins G. Studies in myasthenia gravis. NY State J Med. June 1961; 61:2076-2085.
- Grob D. Myasthenia gravis. A review of pathogenesis and treatment. Arch Intern Med. Oct 1961; 108:615-638.
- Schwab RS. Management of myasthenia gravis. New Eng J Med. Mar 1963; 268:596-597.
- Schwab RS. Management of myasthenia gravis. New Eng J Med. Mar 1963; 268:717-719.
- Cronnelly R, Stanski DR, Miller RD, Sheiner LB. Pyridostigmine kinetics with and without renal function. Clin Pharmacol Ther. 1980; 28:No. 1, 78-81.
- Miller RD. Pharmacodynamics and pharmacokinetics of anticholinesterase. In: Ruegheimer E, Zindler M, ed. Anaesthesiology. (Hamburg, Germany: Congress; Sep 14-21, 1980; 222-223.) (Int Congr. No. 538), Amsterdam, Netherlands: Excerpta Medica; 1981.
- Breyer-Pfaff U, Maier U, Brinkmann AM, Schumm F. Pyridostigmine kinetics in healthy subjects and patients with myasthenia gravis. Clin Pharmacol Ther. 1985; 5:495-501.
Rev. 08-2007
MF # 252-03
Manufactured by:
Corepharma, LLC.
Middlesex, NJ 08846 for
Sandoz Inc.
Princeton, NJ 08540
| PYRIDOSTIGMINE BROMIDE
pyridostigmine bromide tablet |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Labeler - Sandoz Inc (110342024) |
