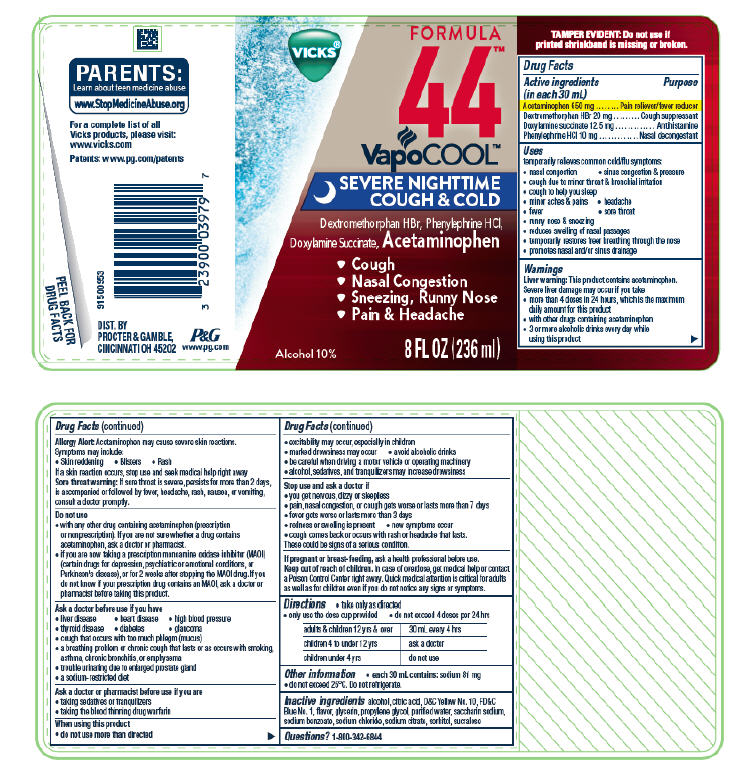
VICKS FORMULA 44 VAPOCOOL SEVERE NIGHTTIME COUGH AND COLD- acetaminophen, dextromethorphan hydrobromide, doxylamine succinate, and phenylephrine hydrochloride solution
The Procter & Gamble Manufacturing Company
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Vicks ® Formula 44™
VapoCool™
Severe Nighttime
Cough & Cold
Acetaminophen 650 mg
Dextromethorphan HBr 20 mg
Doxylamine succinate 12.5 mg
Phenylephrine HCl 10 mg
Uses
temporarily relieves common cold/flu symptoms:
- nasal congestion
- sinus congestion & pressure
- cough due to minor throat & bronchial irritation
- cough to help you sleep
- minor aches & pains
- headache
- fever
- sore throat
- runny nose & sneezing
- reduces swelling of nasal passages
- temporarily restores freer breathing through the nose
- promotes nasal and/or sinus drainage
Warnings
Liver warning
This product contains acetaminophen. Severe liver damage may occur if you take
- more than 4 doses in 24 hours, which is the maximum daily amount for this product
- with other drugs containing acetaminophen
- 3 or more alcoholic drinks every day while using this product
Allergy Alert:
Acetaminophen may cause severe skin reactions. Symptoms may include:
- Skin reddening
- Blisters
- Rash
If a skin reaction occurs, stop use and seek medical help right away
Sore throat warning
If sore throat is severe, persists for more than 2 days, is accompanied or followed by fever, headache, rash, nausea, or vomiting, consult a doctor promptly.
Do not use
- with any other drug containing acetaminophen (prescription or nonprescription). If you are not sure whether a drug contains acetaminophen, ask a doctor or pharmacist.
- if you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric or emotional conditions, or Parkinson's disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product.
- to make a child sleep
Ask a doctor before use if you have
- liver disease
- heart disease
- high blood pressure
- thyroid disease
- diabetes
- glaucoma
- cough that occurs with too much phlegm (mucus)
- a breathing problem or chronic cough that lasts or as occurs with smoking, asthma, chronic bronchitis, or emphysema
- trouble urinating due to enlarged prostate gland
- a sodium-restricted diet
Ask a doctor or pharmacist before use if you are
- taking sedatives or tranquilizers
- taking the blood thinning drug warfarin
When using this product
- do not use more than directed
- excitability may occur, especially in children
- marked drowsiness may occur
- avoid alcoholic drinks
- be careful when driving a motor vehicle or operating machinery
- alcohol, sedatives, and tranquilizers may increase drowsiness
Stop use and ask a doctor if
- you get nervous, dizzy or sleepless
- pain, nasal congestion, or cough gets worse or lasts more than 7 days
- fever gets worse or lasts more than 3 days
- redness or swelling is present
- new symptoms occur
- cough comes back or occurs with rash or headache that lasts.
These could be signs of a serious condition.
Directions
- take only as directed
- only use the dose cup provided
- do not exceed 4 doses per 24 hrs
adults & children 12 yrs & over 30 mL every 4 hrs
children 4 to under 12 yrs ask a doctor
children under 4 yrs do not use
Other information
- each 30 mL dose cup contains: sodium 81 mg
- do not exceed 25°C. Do not refrigerate.
| VICKS FORMULA 44 VAPOCOOL
SEVERE NIGHTTIME COUGH AND COLD
acetaminophen, dextromethorphan hydrobromide, doxylamine succinate, and phenylephrine hydrochloride solution |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Labeler - The Procter & Gamble Manufacturing Company (004238200) |