Label: QUFLORA FE- vitamin a acetate, ascorbic acid, cholecalciferol, .alpha.-tocopherol, dl-, thiamine hydrochloride, riboflavin, niacinamide, pyridoxine hydrochloride, levomefolate glucosamine, cyanocobalamin, biotin, calcium pantothenate, iron pentacarbonyl, zinc oxide, copper, and sodium fluoride tablet, chewable
- NHRIC Code(s): 15370-120-30
- Packager: CarWin Pharmaceutical Associates, LLC
- Category: DIETARY SUPPLEMENT
- DEA Schedule: None
- Marketing Status: Dietary Supplement
Drug Label Information
Updated July 15, 2016
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
STATEMENT OF IDENTITY
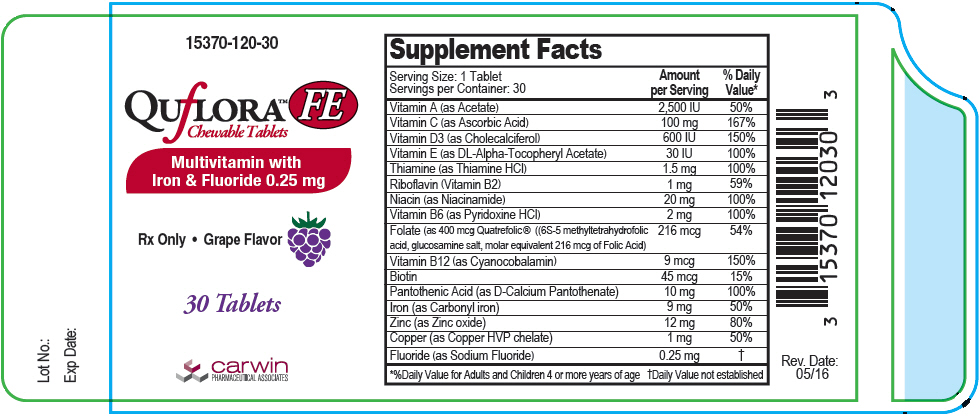
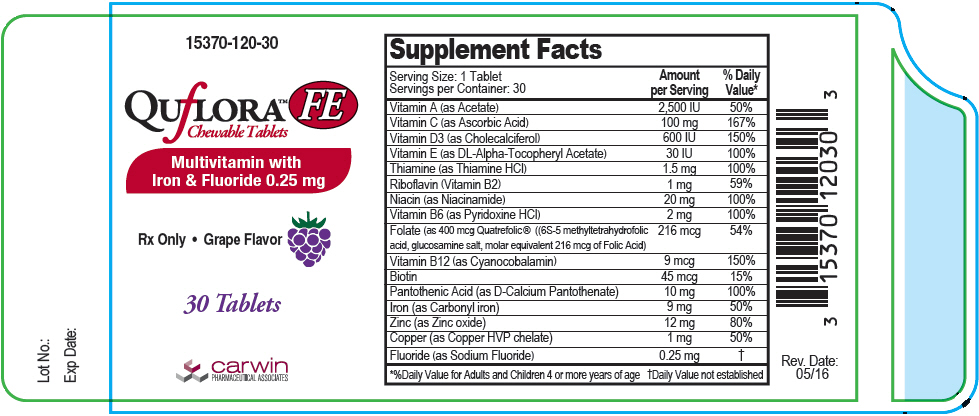
Supplement Facts Serving Size: 1 Tablet
Servings per Container: 30Amount per Serving % Daily Value* Vitamin A (as Acetate) 2500 IU 50% Vitamin C (as Ascorbic Acid) 100 mg 167% Vitamin D3 ( as Cholecalciferol) 600 IU 150% Vitamin E (as DL-Alpha Tocopheryl Acetate) 30 IU 100% Thiamine (as Thiamine HCl) 1.5 mg 100% Riboflavin (Vitamin B2) 1 mg 59% Niacin (as Niacinamide) 20 mg 100% Vitamin B6 (as Pyridoxine HCl) 2 mg 100% Folate (as 400 mcg Quatrefolic® ((6S-5 methyltetrahydrofolic acid, glucosamine salt, molar equivalent 216 mcg of Folic Acid) 216 mcg 54% Vitamin B12 (as Cyanocobalamin) 9 mcg 150% Biotin 45 mcg 15% Pantothenic Acid (as D-Calcium Pantothenate) 10 mg 100% Iron (as Carbonyl iron) 9 mg 50% Zinc (as Zinc oxide) 12 mg 80% Copper (as Copper HVP chelate) 1 mg 50% Fluoride (as Sodium Fluoride) 0.25 mg † Other Ingredients: Citric acid, fumed silica, magnesium stearate, microcrystalline cellulose, natural grape flavor, silicon dioxide, sorbitol, stearic acid, sucralose and xylitol.
- INDICATIONS AND USAGE
- CONTRAINDICATIONS
-
PRECAUTIONS
General
The suggested dose should not be exceeded, since dental fluorosis may result from continued ingestion of large amounts of fluoride. Do not eat or drink dairy products within one hour of fluoride administration. Incompatibility of fluoride with dairy foods has been reported due to formation of calcium fluoride which is poorly absorbed. Your healthcare practitioner can prescribe the correct dosage.
Folic Acid
Folic Acid alone is improper therapy in the treatment of pernicious anemia and other megaloblastic anemias where vitamin B12 is deficient. Folic acid in doses above 0.1 mg daily may obscure pernicious anemia in that hematologic remission can occur while neurological manifestations progress.
- ADVERSE REACTIONS
- WARNINGS
- DOSAGE AND ADMINISTRATION
- DESCRIPTION
- HOW SUPPLIED
- STORAGE
- SAFE HANDLING WARNING
- HEALTH CLAIM
- PRINCIPAL DISPLAY PANEL - 30 Tablet Bottle Label
-
INGREDIENTS AND APPEARANCE
QUFLORA FE
vitamin a acetate, ascorbic acid, cholecalciferol, .alpha.-tocopherol, dl-, thiamine hydrochloride, riboflavin, niacinamide, pyridoxine hydrochloride, levomefolate glucosamine, cyanocobalamin, biotin, calcium pantothenate, iron pentacarbonyl, zinc oxide, copper, and sodium fluoride tablet, chewableProduct Information Product Type DIETARY SUPPLEMENT Item Code (Source) NHRIC:15370-120 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength VITAMIN A ACETATE (UNII: 3LE3D9D6OY) (VITAMIN A - UNII:81G40H8B0T) VITAMIN A 2500 [iU] ASCORBIC ACID (UNII: PQ6CK8PD0R) (ASCORBIC ACID - UNII:PQ6CK8PD0R) ASCORBIC ACID 100 mg CHOLECALCIFEROL (UNII: 1C6V77QF41) (CHOLECALCIFEROL - UNII:1C6V77QF41) CHOLECALCIFEROL 600 [iU] .ALPHA.-TOCOPHEROL, DL- (UNII: 7QWA1RIO01) (.ALPHA.-TOCOPHEROL, DL- - UNII:7QWA1RIO01) .ALPHA.-TOCOPHEROL, DL- 30 [iU] THIAMINE HYDROCHLORIDE (UNII: M572600E5P) (THIAMINE ION - UNII:4ABT0J945J) THIAMINE HYDROCHLORIDE 1.5 mg RIBOFLAVIN (UNII: TLM2976OFR) (RIBOFLAVIN - UNII:TLM2976OFR) RIBOFLAVIN 1 mg NIACINAMIDE (UNII: 25X51I8RD4) (NIACINAMIDE - UNII:25X51I8RD4) NIACINAMIDE 20 mg PYRIDOXINE HYDROCHLORIDE (UNII: 68Y4CF58BV) (PYRIDOXINE - UNII:KV2JZ1BI6Z) PYRIDOXINE HYDROCHLORIDE 2 mg LEVOMEFOLATE GLUCOSAMINE (UNII: Q65PL71Q1A) (LEVOMEFOLATE GLUCOSAMINE - UNII:Q65PL71Q1A) LEVOMEFOLATE GLUCOSAMINE 216 ug CYANOCOBALAMIN (UNII: P6YC3EG204) (CYANOCOBALAMIN - UNII:P6YC3EG204) CYANOCOBALAMIN 9 ug BIOTIN (UNII: 6SO6U10H04) (BIOTIN - UNII:6SO6U10H04) BIOTIN 45 ug CALCIUM PANTOTHENATE (UNII: 568ET80C3D) (PANTOTHENIC ACID - UNII:19F5HK2737, CALCIUM CATION - UNII:2M83C4R6ZB) PANTOTHENIC ACID 10 mg IRON PENTACARBONYL (UNII: 6WQ62TAQ6Z) (FERROUS CATION - UNII:GW89581OWR) FERROUS CATION 9 mg ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 12 mg COPPER (UNII: 789U1901C5) (COPPER - UNII:789U1901C5) COPPER 1 mg SODIUM FLUORIDE (UNII: 8ZYQ1474W7) (FLUORIDE ION - UNII:Q80VPU408O) FLUORIDE ION 0.25 mg Inactive Ingredients Ingredient Name Strength CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) MAGNESIUM STEARATE (UNII: 70097M6I30) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) GRAPE (UNII: 6X543N684K) SORBITOL (UNII: 506T60A25R) STEARIC ACID (UNII: 4ELV7Z65AP) SUCRALOSE (UNII: 96K6UQ3ZD4) XYLITOL (UNII: VCQ006KQ1E) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NHRIC:15370-120-30 30 in 1 BOTTLE, PLASTIC Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date DIETARY SUPPLEMENT 07/12/2016 Supplement Facts Serving Size : Serving per Container : Amount Per Serving % Daily Value color scoring 1 shape size (solid drugs) 14 mm imprint flavor Labeler - CarWin Pharmaceutical Associates, LLC (079217215)