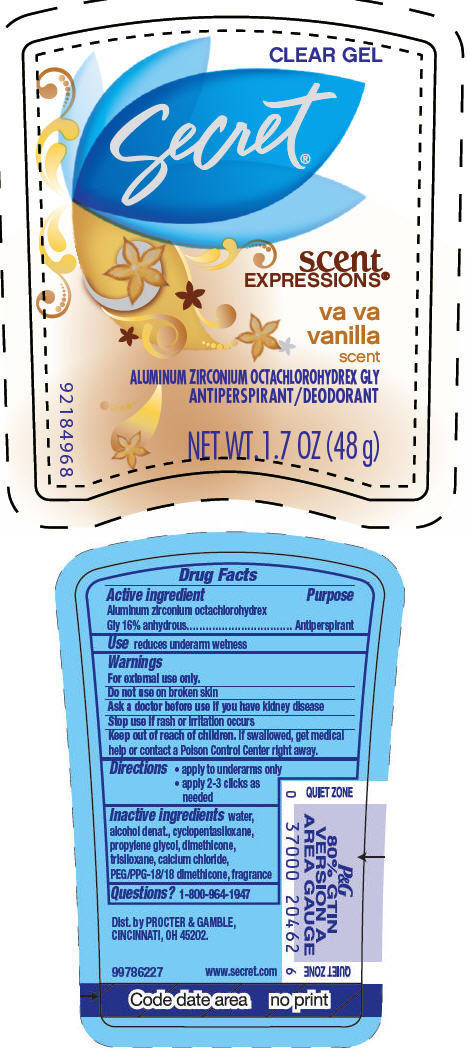
SECRET SCENT EXPRESSIONS CLEAR VA VA VANILLA- aluminum zirconium octachlorohydrex gly gel
Procter & Gamble Manufacturing Company
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Secret®
SCENT
EXPRESSIONS®
| SECRET SCENT EXPRESSIONS
CLEAR VA VA VANILLA
aluminum zirconium octachlorohydrex gly gel |
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| Labeler - Procter & Gamble Manufacturing Company (004238200) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Procter & Gamble Manufacturing Company | 017745779 | MANUFACTURE(37000-211) | |
Revised: 5/2015
Document Id: 8f4b97e4-0f00-4058-a40a-a218f7bb5738
Set id: 6eca5e26-2880-46a9-9afa-6b83315a69f9
Version: 4
Effective Time: 20150501
Procter & Gamble Manufacturing Company