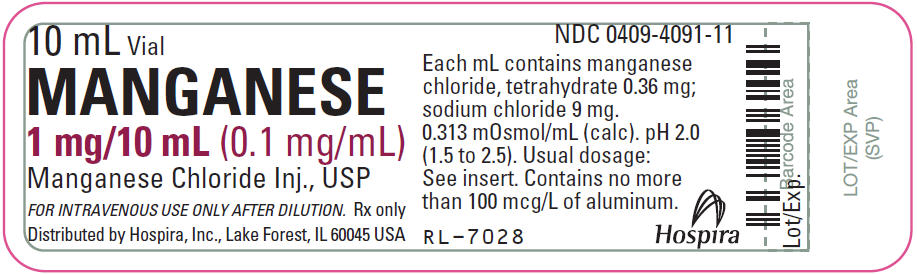
Label: MANGANESE- manganese chloride injection, solution
- NDC Code(s): 0409-4091-01, 0409-4091-11
- Packager: Hospira, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated December 6, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
-
DESCRIPTION
Manganese 0.1 mg/mL (Manganese Chloride Injection, USP) is a sterile, nonpyrogenic solution intended for use as an additive to intravenous solutions for total parenteral nutrition (TPN). Each mL of solution contains 0.36 mg manganese chloride, tetrahydrate and 9 mg sodium chloride. The solution contains no bacteriostat, antimicrobial agent or added buffer. The pH is 2.0 (1.5 to 2.5); product may contain hydrochloric acid and sodium hydroxide for pH adjustment. The osmolarity is 0.313 mOsmol/mL (calc.).
Manganese Chloride, USP is chemically designated manganese chloride, tetrahydrate (MnCl2 • 4H2O), a deliquescent, crystalline compound soluble in water.
Sodium Chloride, USP is chemically designated NaCl, a white, crystalline compound freely soluble in water.
The semi-rigid vial is fabricated from a specially formulated polyolefin. It is a copolymer of ethylene and propylene. The safety of the plastic has been confirmed by tests in animals according to USP biological standards for plastic containers. The small amount of water vapor that can pass through the plastic container wall will not significantly alter the drug concentration.
-
CLINICAL PHARMACOLOGY
Manganese is an essential nutrient which serves as an activator for enzymes such as polysaccharide polymerase, liver arginase, cholinesterase and pyruvate carboxylase. Providing manganese during TPN helps prevent development of deficiency symptoms such as nausea and vomiting, weight loss, dermatitis and changes in growth and color of hair.
Under conditions of minimal intake, 20 mcg manganese/day is retained. Manganese is bound to a specific transport protein, transmanganin, a beta-l-globulin. Manganese is widely distributed but concentrates in the mitochondria rich tissues such as brain, kidney, pancreas, and liver. Assays for manganese in whole blood result in concentrations ranging from 6 to 12 mcg/manganese/liter.
Excretion of manganese occurs mainly through the bile, but in the event of obstruction, ancillary excretion routes include pancreatic juice, or return into the lumen of the duodenum, jejunum, or ileum. Urinary excretion of manganese is negligible.
- INDICATIONS AND USAGE
- CONTRAINDICATIONS
-
WARNINGS
Direct intramuscular or intravenous injection of Manganese 0.1 mg/mL (Manganese Chloride Injection, USP) is contraindicated as the acidic pH of the solution (pH 2.0) may cause considerable tissue irritation.
Neurologic Toxicity with Manganese
Manganese accumulation in the basal ganglia has been reported in adult and pediatric patients on long‑term parenteral nutrition receiving manganese at higher than recommended dosages and in association with cholestatic liver disease. Brain MRI findings and clinical symptoms have also been observed in patients who received manganese at or below the recommended dosage and with normal blood manganese concentrations. Some adult patients with brain MRI findings reportedly experienced neuropsychiatric symptoms, including changes in mood or memory, seizures and/or parkinsonian‑like tremors, dysarthria, mask-face, and halting gait. Some pediatric patients experienced dystonic movements or seizures. Regression of symptoms and MRI findings have occurred over weeks to months following discontinuation of manganese in most patients but have not always completely resolved.
Monitor patients receiving parenteral nutrition solutions containing manganese for neurologic signs and symptoms and routinely measure whole blood manganese concentrations and liver tests. In case of suspected manganese toxicity or new neuropsychiatric manifestations, temporarily discontinue manganese, check manganese whole blood concentrations, and consider brain MRI evaluation.
Hepatic Accumulation of Manganese
Manganese is primarily eliminated in the bile and excretion is decreased in patients with cholestasis and/or cirrhosis. Hepatic accumulation of manganese has been reported in autopsies of patients receiving long-term parenteral nutrition containing manganese at dosages higher than recommended. Patients with cholestasis and/or cirrhosis receiving parenteral nutrition are at increased risk of manganese brain deposition and neurotoxicity. If a patient develops signs or symptoms of hepatobiliary disease during the use of this drug product, obtain manganese whole blood concentrations; consider discontinuing manganese supplementation in these patients until a full clinical evaluation is completed.
Aluminum Toxicity
This product contains aluminum that may be toxic. Aluminum may reach toxic levels with prolonged parenteral administration if kidney function is impaired. Premature neonates are particularly at risk because their kidneys are immature, and they require large amounts of calcium and phosphate solutions, which contain aluminum.
Research indicates that patients with impaired kidney function, including premature neonates, who receive parenteral levels of aluminum at greater than 4 to 5 mcg/kg/day accumulate aluminum at levels associated with central nervous system and bone toxicity. Tissue loading may occur at even lower rates of administration.
-
PRECAUTIONS
General
Do not use unless solution is clear and seal is intact.
Manganese 0.1 mg/mL (Manganese Chloride Injection, USP) should only be used in conjunction with a pharmacy directed admixture program using aseptic technique in a laminar flow environment; it should be used promptly and in a single operation without any repeated penetrations. Solution contains no preservatives; discard unused portion immediately after admixture procedure is completed.
Laboratory Tests
Serum manganese levels can be measured periodically at the discretion of the healthcare provider. Because of the low serum concentration normally present, samples will usually be analyzed by a reference laboratory.
Carcinogenesis, Mutagenesis, and Impairment of Fertility
Long-term animal studies to evaluate the carcinogenic potential of Manganese 0.1 mg/mL (Manganese Chloride Injection, USP) have not been performed, nor have studies been done to assess mutagenesis or impairment of fertility.
Nursing Mothers
It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when Manganese 0.1 mg/mL (Manganese Chloride Injection, USP) additive is administered to a nursing woman.
Pediatric Use
(See DOSAGE AND ADMINISTRATION section.) Safety and effectiveness in pediatric patients have not been established.
Pregnancy
Animal reproduction studies have not been conducted with manganese chloride. It is also not known whether manganese chloride can cause fetal harm when administered to a pregnant woman or can affect reproductive capacity. Manganese chloride should be given to a pregnant woman only if clearly indicated.
Geriatric Use
An evaluation of current literature revealed no clinical experience identifying differences in response between elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
- ADVERSE REACTIONS
- DRUG ABUSE AND DEPENDENCE
-
OVERDOSAGE
Acute manganese toxicity was reported in adult patients following infusion of manganese more than 10,000-fold the recommended dosage and after use of dialysis fluid contaminated with manganese. Signs and symptoms included skin flushing, acute pancreatitis, elevated whole blood manganese concentrations, and MRI evidence of brain accumulation of manganese. Chronic infusion and oral intake of manganese above recommended dosage have resulted in neuropsychiatric symptoms and MRI evidence of brain accumulation of manganese (see WARNINGS).
-
DOSAGE AND ADMINISTRATION
Manganese 0.1 mg/mL (Manganese Chloride Injection, USP) contains 0.1 mg manganese/mL and is administered intravenously only after dilution. The additive should be administered in a volume of fluid at least 100 mL. For the adult receiving TPN, the suggested additive dosage for manganese is 55 mcg/day (0.55 mL/day).
Periodic monitoring of manganese plasma levels is suggested as a guideline for subsequent administration. (See WARNINGS.)
Parenteral products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. (See PRECAUTIONS.)
-
HOW SUPPLIED
Manganese 0.1 mg/mL (Manganese Chloride Injection, USP) is supplied in 10 mL Single-dose Plastic Vials.
Unit of Sale
Concentration
NDC 0409-4091-01
Tray of 25 Single-dose plastic vials1 mg/10 mL
(0.1 mg/mL)Store at 20 to 25°C (68 to 77°F) [See USP Controlled Room Temperature.]
Distributed by Hospira, Inc., Lake Forest, IL 60045 USA
LAB-1068-4.0
Revised: 12/2023
- PRINCIPAL DISPLAY PANEL - 10 mL Vial Label
- PRINCIPAL DISPLAY PANEL - 10 mL Vial Tray
-
INGREDIENTS AND APPEARANCE
MANGANESE
manganese chloride injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0409-4091 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MANGANESE CHLORIDE (UNII: QQE170PANO) (MANGANESE CATION (2+) - UNII:H6EP7W5457) MANGANESE CATION (2+) 0.1 mg in 1 mL Inactive Ingredients Ingredient Name Strength SODIUM CHLORIDE (UNII: 451W47IQ8X) 9 mg in 1 mL HYDROCHLORIC ACID (UNII: QTT17582CB) SODIUM HYDROXIDE (UNII: 55X04QC32I) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0409-4091-01 25 in 1 TRAY 01/31/2005 1 NDC:0409-4091-11 10 mL in 1 VIAL, PLASTIC; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA018962 01/31/2005 Labeler - Hospira, Inc. (141588017) Establishment Name Address ID/FEI Business Operations Hospira, Inc. 093132819 ANALYSIS(0409-4091) , MANUFACTURE(0409-4091) , PACK(0409-4091) , LABEL(0409-4091) Establishment Name Address ID/FEI Business Operations PPD Development, L.P. 838082055 ANALYSIS(0409-4091) Establishment Name Address ID/FEI Business Operations Hospira, Inc. 827731089 ANALYSIS(0409-4091)