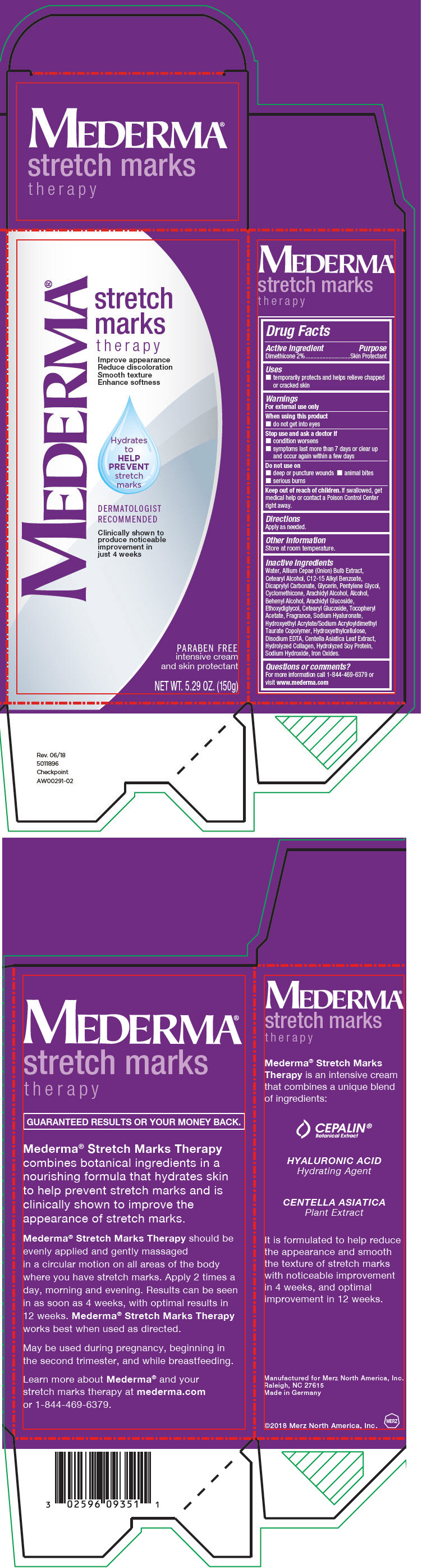
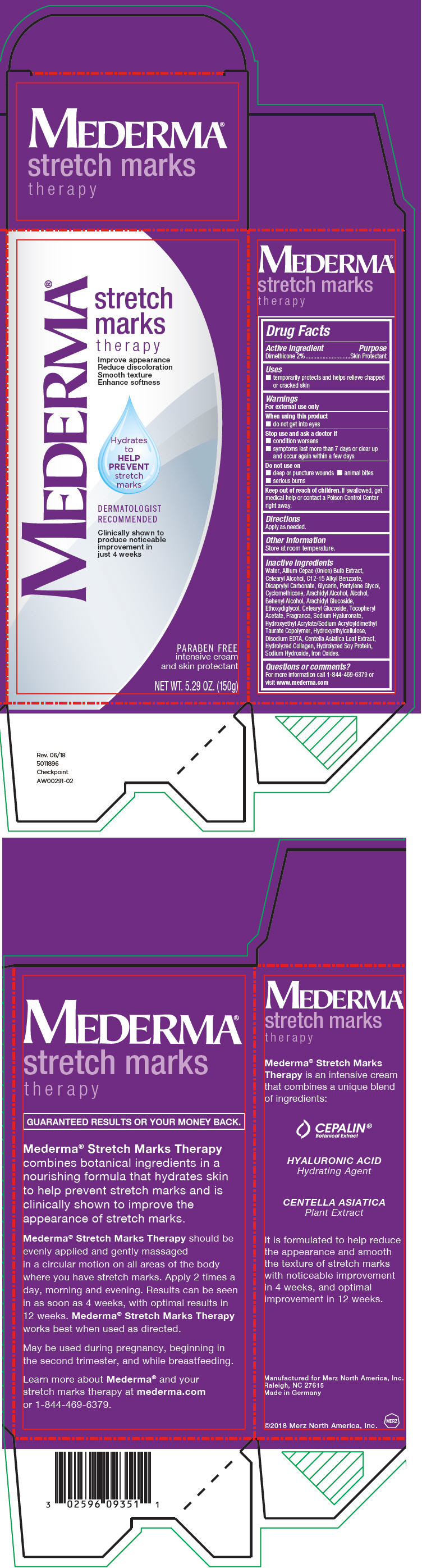
Label: MEDERMA STRETCH MARKS THERAPY- dimethicone cream
- NDC Code(s): 0259-2102-05, 0259-2102-15
- Packager: Merz Pharmaceuticals, LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated February 23, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active Ingredient
- Purpose
- Uses
- Warnings
- Directions
- Other Information
-
Inactive Ingredients
Water, Allium Cepae (Onion) Bulb Extract, Cetearyl Alcohol, C12-15 Alkyl Benzoate, Dicaprylyl Carbonate, Glycerin, Pentylene Glycol, Cyclomethicone, Arachidyl Alcohol, Alcohol, Behenyl Alcohol, Arachidyl Glucoside, Ethoxydiglycol, Cetearyl Glucoside, Tocopheryl Acetate, Fragrance, Sodium Hyaluronate, Hydroxyethyl Acrylate/Sodium Acryloyldimethyl Taurate Copolymer, Hydroxyethylcellulose, Disodium EDTA, Centella Asiatica Leaf Extract, Hydrolyzed Collagen, Hydrolyzed Soy Protein, Sodium Hydroxide, Iron Oxides.
- Questions or comments?
- PRINCIPAL DISPLAY PANEL - 150 g Tube Box
-
INGREDIENTS AND APPEARANCE
MEDERMA STRETCH MARKS THERAPY
dimethicone creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:0259-2102 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength dimethicone (UNII: 92RU3N3Y1O) (dimethicone - UNII:92RU3N3Y1O) dimethicone 20 mg in 1 g Inactive Ingredients Ingredient Name Strength water (UNII: 059QF0KO0R) Edetate Disodium (UNII: 7FLD91C86K) HYDROXYETHYL CELLULOSE (2000 MPA.S AT 1%) (UNII: S38J6RZN16) ARACHIDYL ALCOHOL (UNII: 1QR1QRA9BU) Docosanol (UNII: 9G1OE216XY) ARACHIDYL GLUCOSIDE (UNII: 6JVW35JOOJ) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) Cetearyl Glucoside (UNII: 09FUA47KNA) Alkyl (C12-15) Benzoate (UNII: A9EJ3J61HQ) Dicaprylyl Carbonate (UNII: 609A3V1SUA) HYDROXYETHYL ACRYLATE/SODIUM ACRYLOYLDIMETHYL TAURATE COPOLYMER (100000 MPA.S AT 1.5%) (UNII: 86FQE96TZ4) FERRIC OXIDE RED (UNII: 1K09F3G675) .Alpha.-Tocopherol Acetate (UNII: 9E8X80D2L0) CYCLOMETHICONE (UNII: NMQ347994Z) ONION (UNII: 492225Q21H) Glycerin (UNII: PDC6A3C0OX) PENTYLENE GLYCOL (UNII: 50C1307PZG) CENTELLA ASIATICA LEAF (UNII: 6810070TYD) DIETHYLENE GLYCOL MONOETHYL ETHER (UNII: A1A1I8X02B) BOVINE TYPE I COLLAGEN (UNII: FHJ3ATL51C) SOY PROTEIN (UNII: R44IWB3RN5) HYALURONATE SODIUM (UNII: YSE9PPT4TH) SODIUM HYDROXIDE (UNII: 55X04QC32I) Product Characteristics Color WHITE Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0259-2102-15 1 in 1 BOX 12/01/2013 06/30/2024 1 150 g in 1 TUBE; Type 0: Not a Combination Product 2 NDC:0259-2102-05 0.5 g in 1 PACKET; Type 0: Not a Combination Product 12/01/2013 06/30/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part347 12/01/2013 06/30/2024 Labeler - Merz Pharmaceuticals, LLC (126209282)