Label: ANTIBACTERIAL BANDAGES- benzalkonium chloride dressing
-
NDC Code(s):
71584-0108-1,
71584-0108-2,
71584-0108-3,
71584-0108-4, view more71584-0108-5, 71584-0108-6
- Packager: Guangdong Comfort Medical Products Co., Ltd.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated March 1, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENTS
- Purpose
- USE
-
WARNINGS
For external use only.
Stop use and consult a doctor if the condition persists or gets worse.
Do not use longer than 1 week unless directed by a physician. In case of deep or puncture wounds, animal bites, or serious burn, consult a physician.
Keep out of reach of children. if swallowed get medical help or contact Poison control Center right away.
- DIRECTIONS
- Other Information
- INACTIVE INGREDIENT
- Keep out of reach of children
- Dosage and administration
-
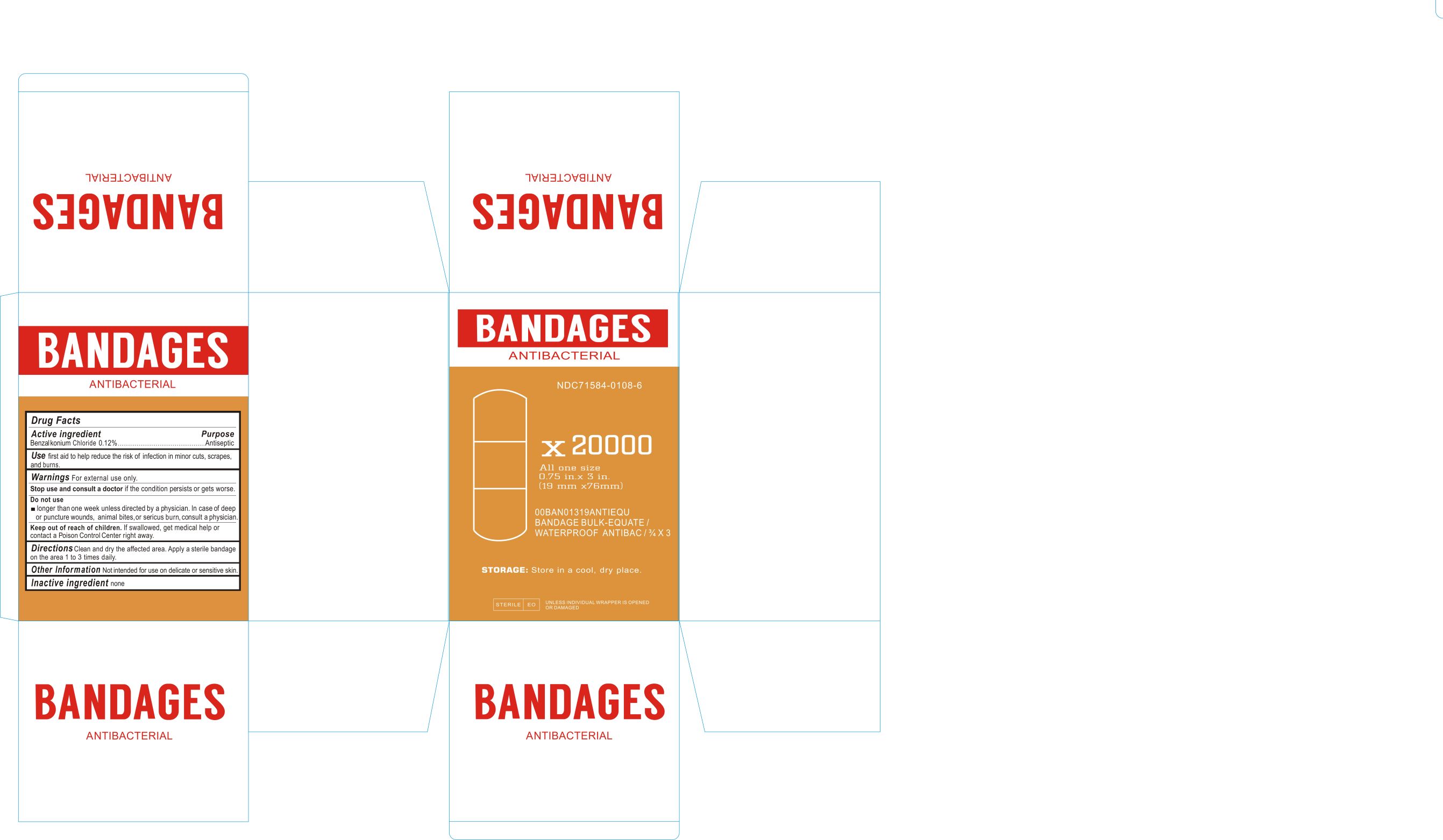
Label principal display panel
NDC 71584-0108-1
ANTIBACTERIAL BANDAGES
Drug Facts
ACTIVE INGREDIENTS
Benzalkonium Chloride 0.12%Purpose
AntisepticUSE
First aid to help reduce the risk of infection in minor cuts, scrapes, and burns.WARNINGS For external use only.
Stop use and consult a doctor if the condition persists or gets worse.
Do not use
longer than one week unless directed by a physician. In case of deep or puncture wounds, animal bites, or serious burn, consult a physician.Keep out of reach of children. if swallowed get medical help or contact Poison control Center right away.
DIRECTIONS
Clean and dry the affected area. Apply a sterile bandage on the area 1 to 3 times daily.Other Information: not intended for use on delicate or sensitive skin.
INACTIVE INGREDIENT
None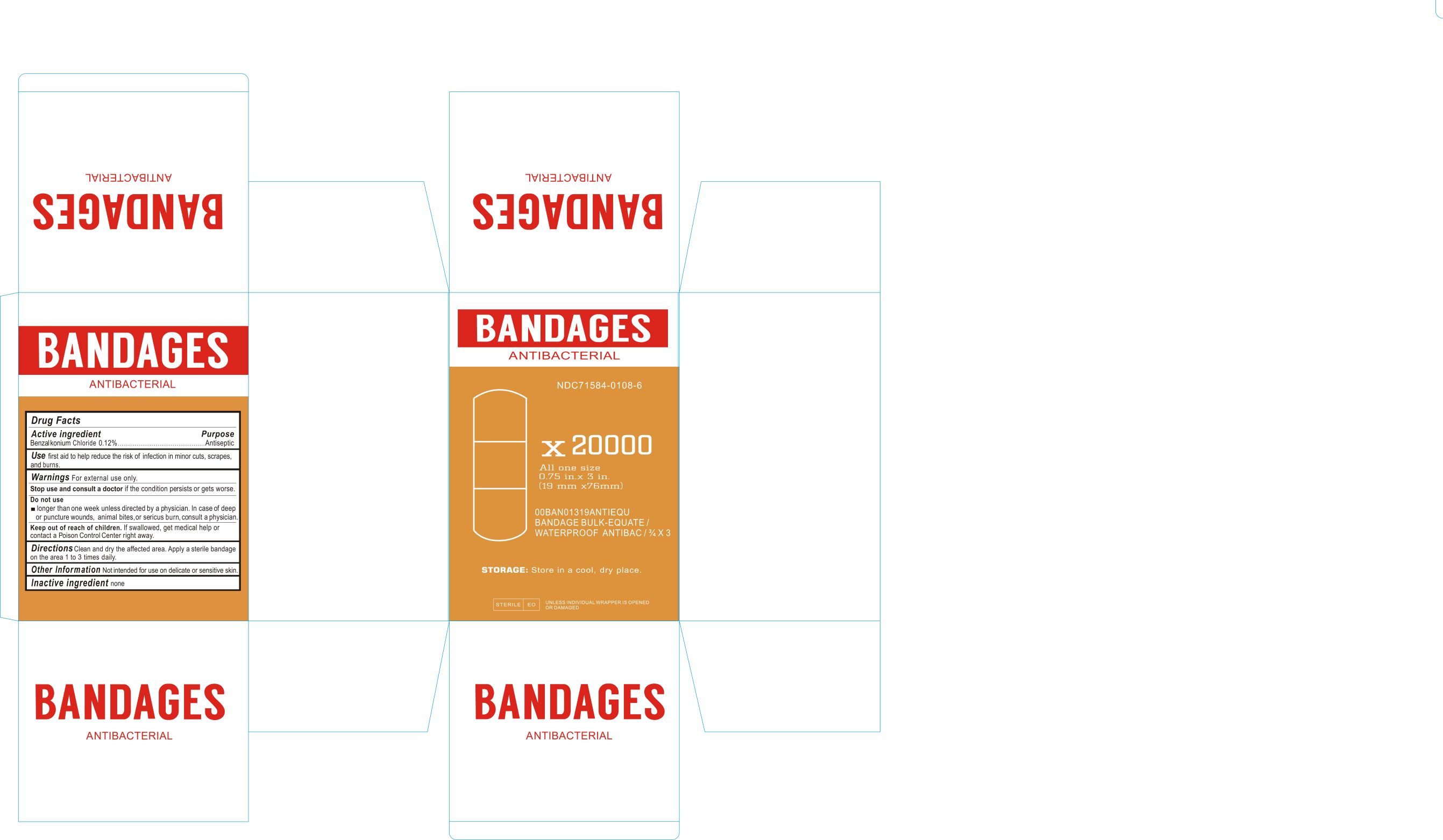









-
INGREDIENTS AND APPEARANCE
ANTIBACTERIAL BANDAGES
benzalkonium chloride dressingProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:71584-0108 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) (BENZALKONIUM - UNII:7N6JUD5X6Y) BENZALKONIUM CHLORIDE 0.12 g in 100 g Inactive Ingredients Ingredient Name Strength COTTON FIBER (UNII: 70LDW53ROO) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:71584-0108-1 0.5 g in 1 POUCH; Type 0: Not a Combination Product 06/10/2018 2 NDC:71584-0108-2 0.5 g in 1 POUCH; Type 0: Not a Combination Product 10/05/2021 3 NDC:71584-0108-3 0.5 g in 1 POUCH; Type 0: Not a Combination Product 10/05/2021 4 NDC:71584-0108-4 0.5 g in 1 POUCH; Type 0: Not a Combination Product 10/05/2021 5 NDC:71584-0108-5 0.5 g in 1 POUCH; Type 0: Not a Combination Product 10/05/2021 6 NDC:71584-0108-6 0.5 g in 1 POUCH; Type 0: Not a Combination Product 10/05/2021 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part333A 06/10/2018 Labeler - Guangdong Comfort Medical Products Co., Ltd. (544507534) Registrant - Guangdong Comfort Medical Products Co., Ltd. (544507534) Establishment Name Address ID/FEI Business Operations Guangdong Comfort Medical Products Co., Ltd. 544507534 manufacture(71584-0108)