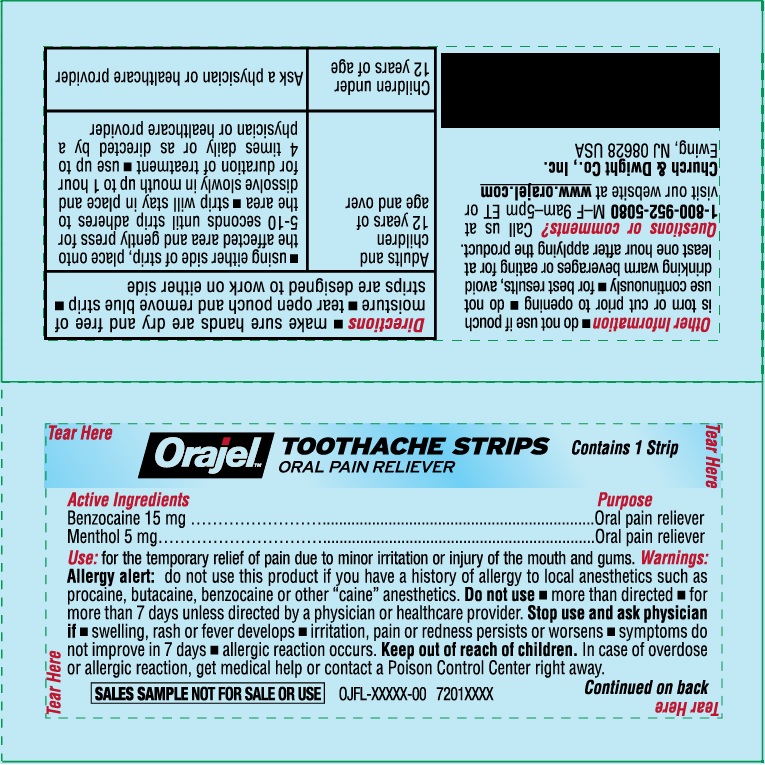
ORAJEL TOOTHACHE STRIPS- benzocaine, menthol film, soluble
BioFilm Limited
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Orajel Toothache Strips
Warnings
Allergy alert: do not use this product if you have a history of allergy to local anesthetics such as procaine, butacaine, benzocaine or other "caine" anesthetics
Do not use
- more than directed
- for more than 7 days unless directed by a physician or healthcare provider
Directions
- make sure hands are dry and free of moisture
- tear open puch and remove blue stip
- strips are desinged to work on either side
| Adults and children 12 years of age and over |
|
| Children under 12 years of age | Ask a physician or healthcare provider |
Other information
- do not use if pouch is torn or cut prior to opening
- do not use continuously
- for best results, avoid drinking warm beverages or eating for at least one hour after applying the product
Inactive ingredients
acid blue 9, glycerin PEG-8, PEG-90, polycarbophil, potassium sorbate, pullulan, PVP, titanium dioxide, water. Formula subject to change.
| ORAJEL TOOTHACHE STRIPS
benzocaine, menthol film, soluble |
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
| Labeler - BioFilm Limited (734555951) |
Revised: 12/2018
Document Id: 7c36fc05-5242-74b5-e053-2991aa0a4e6d
Set id: 6e10252c-3c52-7231-e053-2991aa0a2502
Version: 2
Effective Time: 20181204
BioFilm Limited