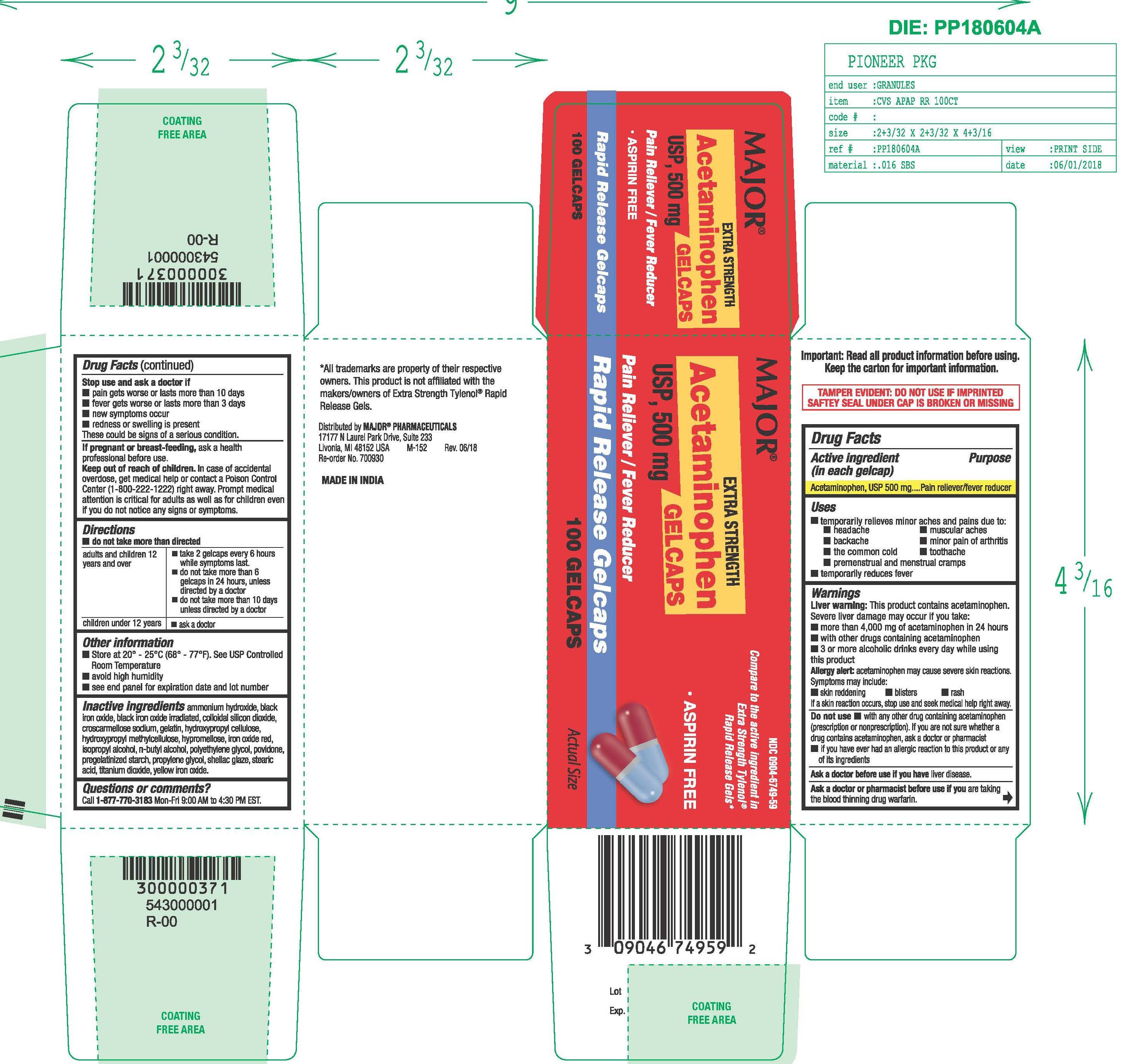
Label: ACETAMINOPHEN tablet
-
Contains inactivated NDC Code(s)
NDC Code(s): 0904-6749-59 - Packager: MAJOR PHARMACEUTICALS
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated January 8, 2021
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredient
- Purpose
- Uses
- Liver warning
- Allergy alert
- Do not use
- Ask a doctor before use if you have
- Ask a doctor or pharmacist before use if you are
- Stop use and ask a doctor if
- If pregnant or breast-feeding,
- Keep out of the reach of children.
- Directions
- Other information
-
Inactive ingredients
ammonium hydroxide, black iron oxide, black iron oxide irradiated, colloidal silicon dioxide, croscarmellose sodium, gelatin, hydroxypropyl cellulose, hydroxypropyl methyl cellulose, hypromellose, iron oxide red, isopropyl alcohol, n-butyl alcohol, polyethylene glycol, povidone, pregelatinized starch, propylene glycol, shellac glaze, stearic acid, titanium dioxide, yellow iron oxide.
- Questions or comments?
- Acetaminophen, USP 500 mg Rapid Release Gelcaps
-
INGREDIENTS AND APPEARANCE
ACETAMINOPHEN
acetaminophen tabletProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:0904-6749 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ACETAMINOPHEN (UNII: 362O9ITL9D) (ACETAMINOPHEN - UNII:362O9ITL9D) ACETAMINOPHEN 500 mg Inactive Ingredients Ingredient Name Strength AMMONIA (UNII: 5138Q19F1X) GELATIN (UNII: 2G86QN327L) FERRIC OXIDE RED (UNII: 1K09F3G675) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) HYDROXYPROPYL CELLULOSE, UNSPECIFIED (UNII: 9XZ8H6N6OH) ISOPROPYL ALCOHOL (UNII: ND2M416302) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) SHELLAC (UNII: 46N107B71O) FERROSOFERRIC OXIDE (UNII: XM0M87F357) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) HYPROMELLOSES (UNII: 3NXW29V3WO) BUTYL ALCOHOL (UNII: 8PJ61P6TS3) POVIDONE (UNII: FZ989GH94E) STARCH, PREGELATINIZED CORN (UNII: O8232NY3SJ) STEARIC ACID (UNII: 4ELV7Z65AP) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) Product Characteristics Color gray (Encapsulated with red opaque and blue gray opaque hard gelatin shells) Score no score Shape OVAL Size 19mm Flavor Imprint Code G1 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0904-6749-59 100 in 1 BOTTLE; Type 0: Not a Combination Product 10/15/2018 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part343 10/15/2018 Labeler - MAJOR PHARMACEUTICALS (191427277)