VIRT VITE GT- .beta.-carotene, ascorbic acid, cholecalciferol, .alpha.-tocopherol acetate, dl-, thiamine mononitrate, riboflavin, niacinamide, pyridoxine hydrochloride, folic acid, cyanocobalamin, biotin, calcium pantothenate, calcium carbonate, iron, magnesium oxide, zinc oxide, cupric oxide and docusate tablet
Virtus Pharmaceuticals OpCo II
----------
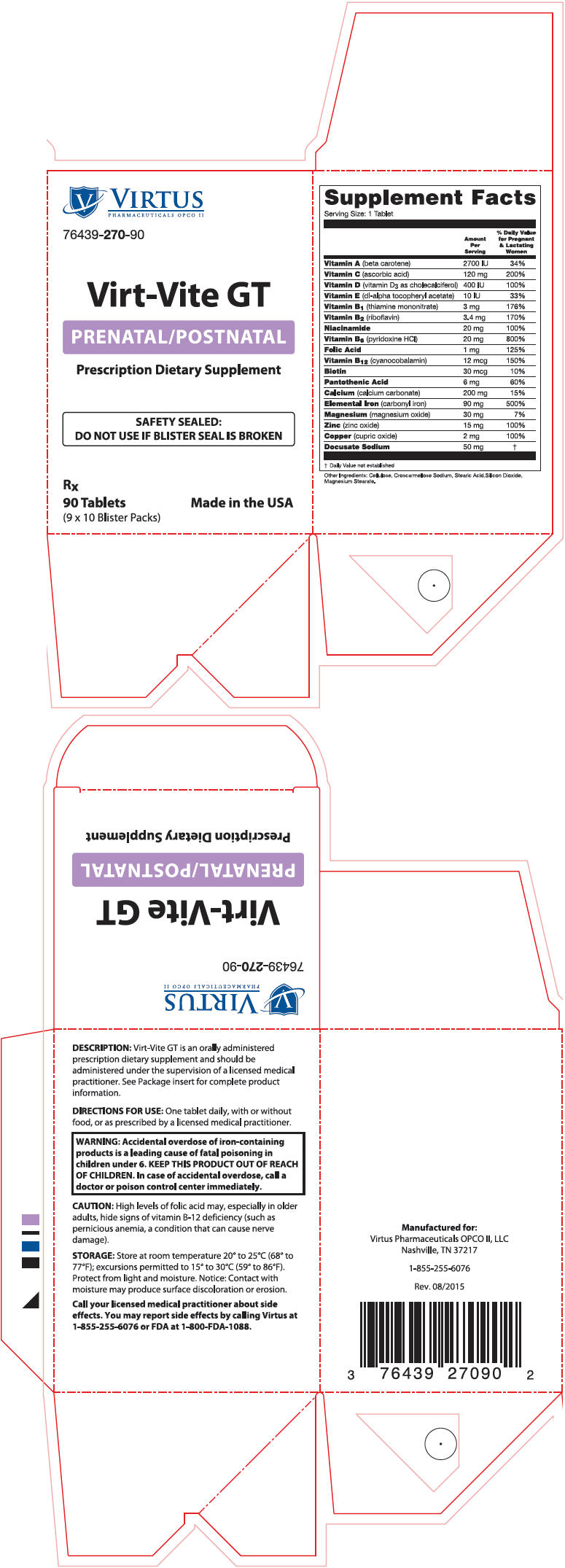
Virt-Vite GT
DESCRIPTION
Virt-Vite GT is an orally administered prescription dietary supplement and should be administered under the supervision of a licensed medical practitioner.
DIRECTIONS FOR USE
One tablet daily with or without food or as prescribed by a licensed medical practitioner.
| Supplement Facts Serving Size: 1 Tablet |
||
|---|---|---|
| Amount Per Serving | % Daily Value for Pregnant & Lactating Women | |
|
||
| Vitamin A (beta carotene) | 2700 IU | 34% |
| Vitamin C (ascorbic acid) | 120 mg | 200% |
| Vitamin D (vitamin D3 as cholecalciferol) | 400 IU | 100% |
| Vitamin E (di-alpha tocopheryl acetate) | 10 IU | 33% |
| Vitamin B1 (thiamine mononitrate) | 3 mg | 176% |
| Vitamin B2 (riboflavin) | 3.4 mg | 170% |
| Niacinamide | 20 mg | 100% |
| Vitamin B6 (pyridoxine HCl) | 20 mg | 800% |
| Folic Acid | 1 mg | 125% |
| Vitamin B12 (cyanocobalamin) | 12 mcg | 150% |
| Biotin | 30 mcg | 10% |
| Pantothenic Acid | 6 mg | 60% |
| Calcium (calcium carbonate) | 200 mg | 15% |
| Elemental Iron (carbonyl iron) | 90 mg | 500% |
| Magnesium (magnesium oxide) | 30 mg | 7% |
| Zinc (zinc oxide) | 15 mg | 100% |
| Copper (cupric oxide) | 2 mg | 100% |
| Docusate Sodium | 50 mg | * |
Other Ingredients: Cellulose, Croscarmellose Sodium, Stearic Acid, Silicon Dioxide, Magnesium Stearate.
KEEP THIS OUT OF REACH OF CHILDREN
CONTRAINDICATIONS
This product is contraindicated for persons with known hypersensitivity to any of its components.
Products containing iron should not be used during dimercaprol therapy.
This product should not be used with laxatives.
| WARNING: Accidental overdose of iron-containing products is a leading cause of fatal poisoning in children under 6. KEEP THIS PRODUCT OUT OF REACH OF CHILDREN. In case of accidental overdose, call a doctor or poison control center immediately. |
CAUTION
High levels of folic acid may, especially in older adults, hide signs of vitamin B-12 deficiency (such as pernicious anemia, a condition that can cause nerve damage).
SIDE EFFECTS
Hypersensitivity side effects have been reported rarely for folic acid. They have included erythema, rash, pruritis, malaise, dyspnea with bronchospasm.
Thiamine mononitrate and riboflavin do not produce side effects. Riboflavin may cause urine to become more yellow than usual.
Oral pyridoxine is considered to be safe. Hypersensitivity, photosensitivity, and allergic dermatitis have been reported rarely.
Orally administered vitamin B12 has no reported side effects.
Carbonyl Iron side effects are most frequently reported to be gastrointestinal. They have included diarrhea, cramping, nausea, constipation, heartburn, and epigastric discomfort. Other side effects have included iron overload (hemosiderosis) and stained teeth. Headaches have been reported occasionally.
In the Virt-Vite GT formulation, zinc oxide is considered to be safe; It has been reported to be "probably safe" when as much as 40 mg per day is ingested. Zinc oxide overdose causes gastrointestinal distress; abdominal pain, nausea, bloating, gas, and diarrhea may occur. Fever and chills can also signify inappropriate exposure.
DRUG INTERACTIONS
Ascorbic acid may decrease the effects of amphetamine. Preliminary data suggest that ascorbic acid may diminish the antitumor effects of bortezomib when taken simultaneously.
Riboflavin absorption can be increased by concurrent use of anticholinergic drugs. The clinical significance of this has not been determined.
Pyridoxine alters the duration of response to the antineoplastic drug altretamine. Some antiepileptic medications, including valproic acid, carbamazepine, phenobarbital, and phenytoin increase the catabolism rate of vitamin B6 resulting in hyperhomocysteinemia. The action of levodopa is potentiated by pyridoxine.
Folic acid has been reported to potentiate the pharmacologic effects of 5-fluorouracil (5-FU) (and its prodrugs capecitabine and tegafur) especially if prescribed in combination with leucovorin. Co-administration with folate therapy may reduce the anticonvulsant effects of phenytoin, phenobarbital, primidone, and succinimides.
Carbonyl iron is known to interact with dolutegravir (may decrease the oral bioavailability). Dimercaprol can bind with iron, forming a complex that is toxic to the kidneys. Carbonyl iron may decrease the absorption of tetracycline antibiotics, fluoroquinolone antibiotics, levodopa, methyldopa, levothyroxine products, or penicillamine.
Orally administered zinc oxide may interfere with the absorption of quinolone or tetracycline antibiotics.
HEALTH CLAIM
Adequate folate in healthful diets may reduce a woman's risk of having a child with a brain or spinal cord birth defect.
HOW SUPPLIED
Supplied as oval, light purple-coated tablets debossed with "V270" dispensed in boxes of 90 tablets (9 × 10 blister packs).
| VIRT VITE GT
.beta.-carotene, ascorbic acid, cholecalciferol, .alpha.-tocopherol acetate, dl-, thiamine mononitrate, riboflavin, niacinamide, pyridoxine hydrochloride, folic acid, cyanocobalamin, biotin, calcium pantothenate, calcium carbonate, iron, magnesium oxide, zinc oxide, cupric oxide and docusate tablet |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Supplement Facts | ||
| Serving Size : | Serving per Container : | |
| Amount Per Serving | % Daily Value | |
|---|---|---|
| color | ||
| scoring | 1 | |
| shape | ||
| size (solid drugs) | 19 mm | |
| imprint | ||
| Labeler - Virtus Pharmaceuticals OpCo II (969483143) |