MEDLINE READYBATH LUXE ANTIBACTERIAL FORMULA- benzalkonium chloride cloth
MEDLINE READYBATH LUXE ANTIBACTERIAL FORMULA FRAGRANCE FREE- benzalkonium chloride cloth
MEDLINE READYBATH SELECT ANTIBACTERIAL FORMULA- benzalkonium chloride cloth
MEDLINE READYBATH SELECT ANTIBACTERIAL FORMULA FRAGRANCE FREE- benzalkonium chloride cloth
Medline Industries, Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Active Ingredient
Benzalkonium Chloride 0.12%
Uses
- For body cleansing to decrease bacteria on the skin
- Helps kill germs that can cause odor
Warnings
For external use only
Ask a doctor before use if you have
- deep or puncture wounds
- animal bites
- serious burns
Stop use and ask a doctor if
- irritation and redness develop
- the condition persists for more than 72 hours or gets worse
Keep out of reach of children.
If swallowed get medical help or contact a Poison Control Center right away.
Directions
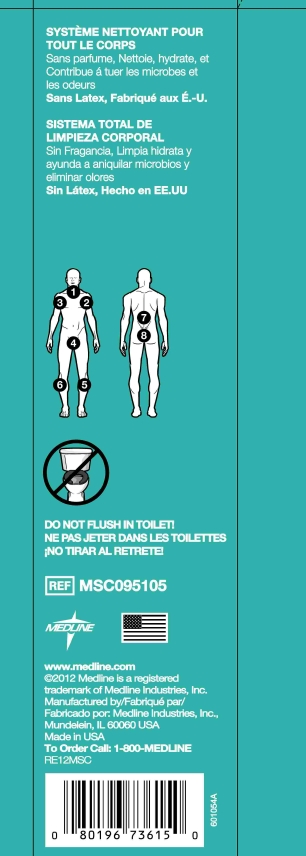
Washcloths can be used at room temperature or heated in a microwave for extra comfort.
- Gently pull label tab until package opening is exposed
- Remove cloths one at a time to cleanse body parts in the order of the diagram below
- Allow skin to dry
- Dispose of cloth in waste receptacle
- Do not flush cloths in toilet
Other information
- Avoid excessive heat. Protect from freezing.
- Washcloth: rayon/polyester
Inactive ingredients
Allantoin, Aloe Barbadensis Leaf Juice, Citric Acid, Cocamidopropyl PG-Dimonium Chloride Phosphate, Disodium EDTA, Fragrance, Linoleamidopropyl PG-Dimonium Chloride Phosphate, Polysorbate 20, Sodium Benzoate, Simethicone, Water (Aqua)
Package Label - Principal Display Panel
Package Label - Principal Display Panel
Package Label - Principal Display Panel
Package Label - Principal Display Panel
Package Label - Package back left
Package Label - Package back right
Medline Industries, Inc.