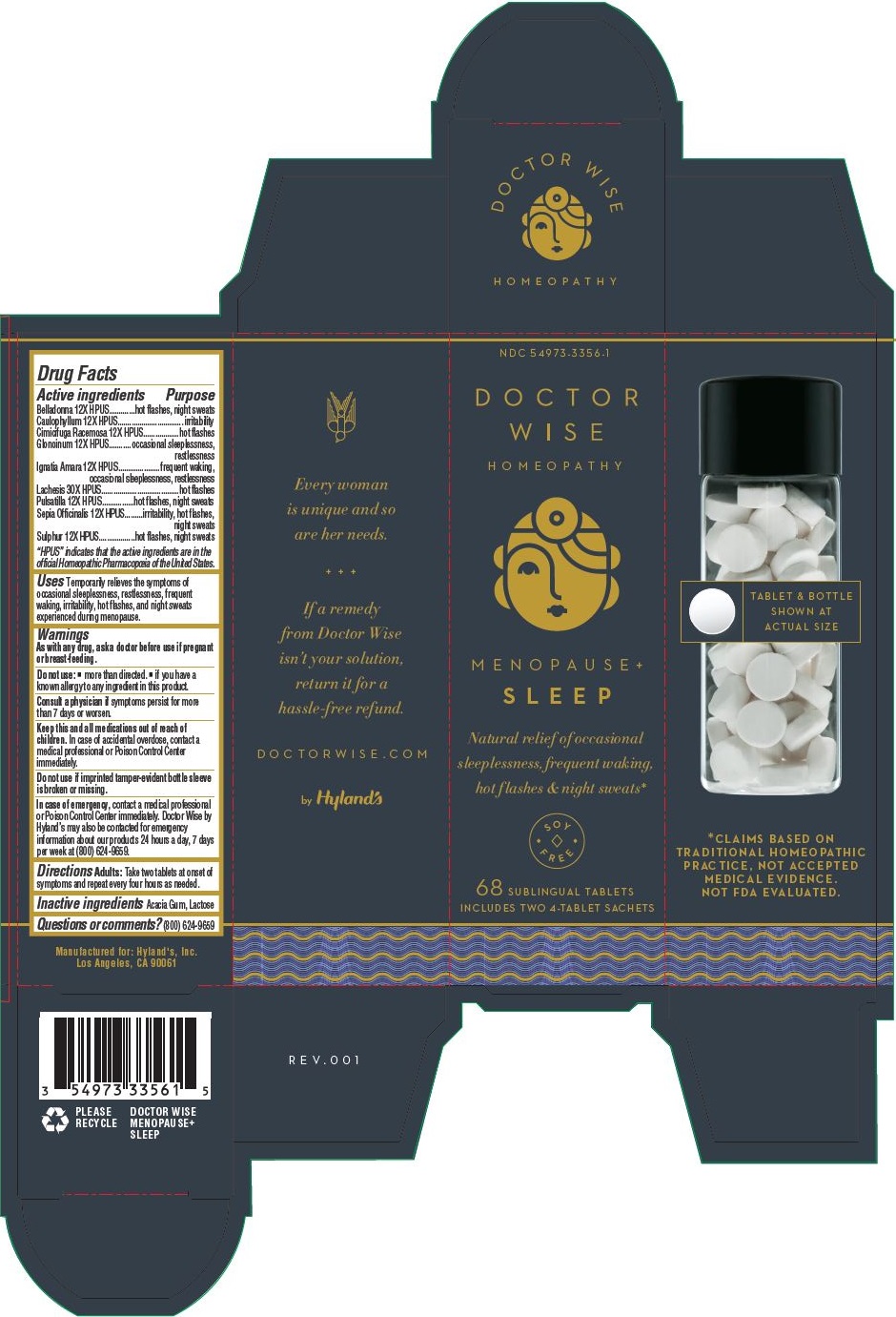
Label: MENOPAUSE PLUS SLEEP- atropa belladonna, caulophyllum thalictroides root, black cohosh, nitroglycerin, strychnos ignatii seed, anemone pulsatilla, sepia officinalis juice, sulfur and lachesis muta venom kit
- NDC Code(s): 54973-3355-1, 54973-3355-2, 54973-3356-1
- Packager: Hyland's Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved homeopathic
DISCLAIMER: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Drug Label Information
Updated December 13, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
Drug Facts
Active ingredients
Active ingredients
Purpose
Belladonna 12X HPUS
hot flashes, night sweats
Caulophyllum 12X HPUS
irritability
Cimicifuga Racemosa 12X HPUS
hot flashes
Glonoinum 12X HPUS
occasional sleeplessness, restlessness
Ignatia Amara 12X HPUS
frequent waking, occasional sleeplessness, restlessness
Lachesis 30X HPUS
hot flashes
Pulsatilla 12X HPUS
hot flashes, night sweats
Sepia Officinalis 12X HPUS
irritability, hot flashes, night sweats
Sulphur 12X HPUS
hot flashes, night sweats
“HPUS” indicates that the active ingredients are in the official Homeopathic Pharmacopœia of the United States.
- Uses
- Warnings
- Directions
- Inactive ingredients
- Questions or comments?
- Principal Display Panel - Doctor Wise Menopause+ Sleep
-
INGREDIENTS AND APPEARANCE
MENOPAUSE PLUS SLEEP
atropa belladonna, caulophyllum thalictroides root, black cohosh, nitroglycerin, strychnos ignatii seed, anemone pulsatilla, sepia officinalis juice, sulfur and lachesis muta venom kitProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:54973-3356 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:54973-3356-1 1 in 1 CARTON; Type 0: Not a Combination Product 06/01/2018 Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 PACKET 4 Part 2 1 BOTTLE, PLASTIC 60 Part 1 of 2 MENOPAUSE PLUS SLEEP
nitroglycerin, strychnos ignatii seed, anemone pulsatilla, black cohosh, lachesis muta venom, sepia officinalis juice, sulfur, atropa belladonna and caulophyllum thalictroides root tabletProduct Information Item Code (Source) NDC:54973-3355 Route of Administration SUBLINGUAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength STRYCHNOS IGNATII SEED (UNII: 1NM3M2487K) (STRYCHNOS IGNATII SEED - UNII:1NM3M2487K) STRYCHNOS IGNATII SEED 12 [hp_X] ATROPA BELLADONNA (UNII: WQZ3G9PF0H) (ATROPA BELLADONNA - UNII:WQZ3G9PF0H) ATROPA BELLADONNA 12 [hp_X] SEPIA OFFICINALIS JUICE (UNII: QDL83WN8C2) (SEPIA OFFICINALIS JUICE - UNII:QDL83WN8C2) SEPIA OFFICINALIS JUICE 12 [hp_X] SULFUR (UNII: 70FD1KFU70) (SULFUR - UNII:70FD1KFU70) SULFUR 12 [hp_X] ANEMONE PULSATILLA (UNII: I76KB35JEV) (ANEMONE PULSATILLA - UNII:I76KB35JEV) ANEMONE PULSATILLA 12 [hp_X] CAULOPHYLLUM THALICTROIDES ROOT (UNII: JTJ6HH6YEH) (CAULOPHYLLUM THALICTROIDES ROOT - UNII:JTJ6HH6YEH) CAULOPHYLLUM THALICTROIDES ROOT 12 [hp_X] BLACK COHOSH (UNII: K73E24S6X9) (BLACK COHOSH - UNII:K73E24S6X9) BLACK COHOSH 12 [hp_X] NITROGLYCERIN (UNII: G59M7S0WS3) (NITROGLYCERIN - UNII:G59M7S0WS3) NITROGLYCERIN 12 [hp_X] LACHESIS MUTA VENOM (UNII: VSW71SS07I) (LACHESIS MUTA VENOM - UNII:VSW71SS07I) LACHESIS MUTA VENOM 30 [hp_X] Inactive Ingredients Ingredient Name Strength ACACIA (UNII: 5C5403N26O) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) Product Characteristics Color white (White to off-white) Score no score Shape ROUND Size 9mm Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:54973-3355-2 4 in 1 PACKET; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 06/01/2018 Part 2 of 2 MENOPAUSE PLUS SLEEP
nitroglycerin, strychnos ignatii seed, anemone pulsatilla, black cohosh, lachesis muta venom, sepia officinalis juice, sulfur, atropa belladonna and caulophyllum thalictroides root tabletProduct Information Item Code (Source) NDC:54973-3355 Route of Administration SUBLINGUAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SULFUR (UNII: 70FD1KFU70) (SULFUR - UNII:70FD1KFU70) SULFUR 12 [hp_X] ATROPA BELLADONNA (UNII: WQZ3G9PF0H) (ATROPA BELLADONNA - UNII:WQZ3G9PF0H) ATROPA BELLADONNA 12 [hp_X] CAULOPHYLLUM THALICTROIDES ROOT (UNII: JTJ6HH6YEH) (CAULOPHYLLUM THALICTROIDES ROOT - UNII:JTJ6HH6YEH) CAULOPHYLLUM THALICTROIDES ROOT 12 [hp_X] BLACK COHOSH (UNII: K73E24S6X9) (BLACK COHOSH - UNII:K73E24S6X9) BLACK COHOSH 12 [hp_X] NITROGLYCERIN (UNII: G59M7S0WS3) (NITROGLYCERIN - UNII:G59M7S0WS3) NITROGLYCERIN 12 [hp_X] STRYCHNOS IGNATII SEED (UNII: 1NM3M2487K) (STRYCHNOS IGNATII SEED - UNII:1NM3M2487K) STRYCHNOS IGNATII SEED 12 [hp_X] LACHESIS MUTA VENOM (UNII: VSW71SS07I) (LACHESIS MUTA VENOM - UNII:VSW71SS07I) LACHESIS MUTA VENOM 30 [hp_X] ANEMONE PULSATILLA (UNII: I76KB35JEV) (ANEMONE PULSATILLA - UNII:I76KB35JEV) ANEMONE PULSATILLA 12 [hp_X] SEPIA OFFICINALIS JUICE (UNII: QDL83WN8C2) (SEPIA OFFICINALIS JUICE - UNII:QDL83WN8C2) SEPIA OFFICINALIS JUICE 12 [hp_X] Inactive Ingredients Ingredient Name Strength ACACIA (UNII: 5C5403N26O) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) Product Characteristics Color white (White to off-white) Score no score Shape ROUND Size 9mm Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:54973-3355-1 60 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 06/01/2018 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 06/01/2018 Labeler - Hyland's Inc. (008316655) Establishment Name Address ID/FEI Business Operations Hyland's Inc. 008316655 manufacture(54973-3356) , pack(54973-3356) , label(54973-3356) Establishment Name Address ID/FEI Business Operations Merical, Inc 118445308 manufacture(54973-3356)