Label: PRENATVITE tablet
- NHRIC Code(s): 73317-1050-9
- Packager: SLV PHARMACEUTICALS LLC DBA AUM PHARMACEUTICALS
- Category: DIETARY SUPPLEMENT
- DEA Schedule: None
- Marketing Status: Dietary Supplement
Drug Label Information
Updated February 5, 2020
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
DESCRIPTION
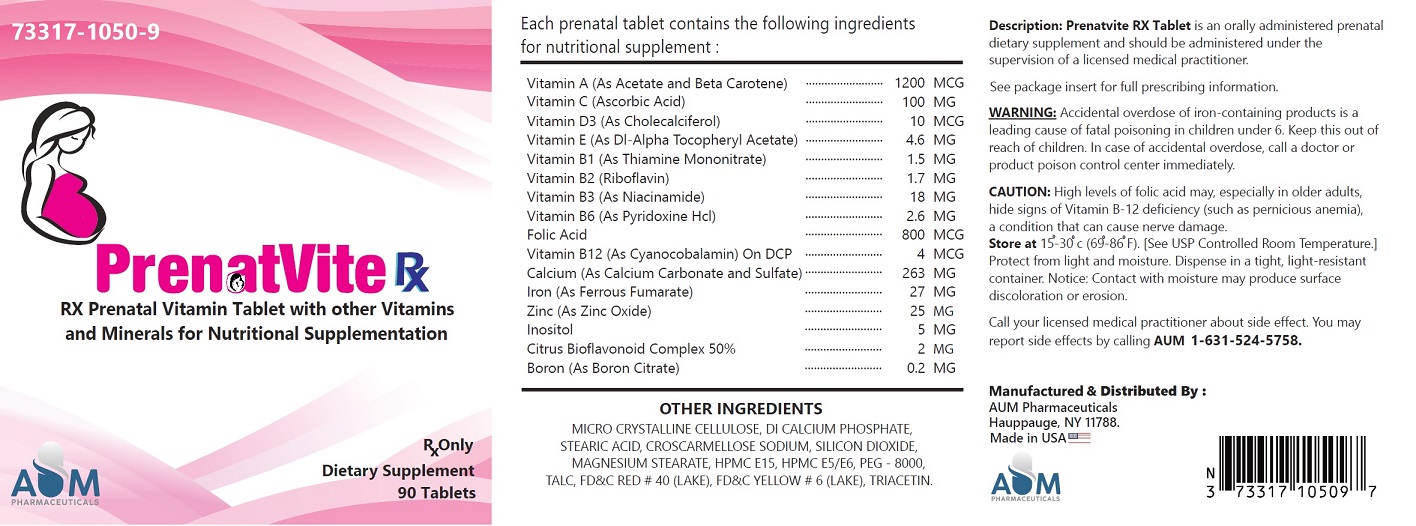
SUPPLEMENT FACTS:
Serving Size: One Tablet
PRENATVITE RX is an orally administered prenatal dietary Supplement and should be administered under the supervision of a licensed medical practitioner.
Each PRENATVITE tablet contains:
VITAMIN A (AS ACETATE, BETA CAROTENE)
1200 MCG
VITAMIN C (ASCORBIC ACID)
120 MG VITAMIN D3 (AS CHOLECALCIFEROL)
10 MCG
VITAMIN E (AS DL-ALPHA TOCOPHEROL ACETATE)
4.6 MG
VITAMIN B1 (AS THIAMINE MONONITRATE)
1.5 MG VITAMIN B2 (AS RIBOFLAVIN) 1.7 MG
VITAMIN B3 (AS NIACINAMIDE)
18 MG
VITAMIN B6 (AS PYRIDOXINE HCL)
2.6 MG
FOLIC ACID
800 MCG
VITAMIN B12 (AS CYANOCOBALAMIN)
4 MCG
CALCIUM (AS CALCIUM CARBONATE & SULFATE)
263 MG
IRON (AS FERROUS FUMARATE)
27 MG
ZINC (AS ZINC OXIDE)
25 MG
INOSITOL
5 MG
CITRUS BIOFLAVONOIDS COMPLEX 50%
2 MG
BORON (AS BORON CITRATE)
0.2 MG
Other Ingredients:
MICRO CRYSTALLINE CELLULOSE, DI CALCIUM PHOSPHATE, STEARIC ACID, CROSCARMELLOSE SODIUM, SILICON DIOXIDE, MAGNESIUM STEARATE, HPMC E15, HPMC E5/E6, PEG - 8000, TALC, FD&C RED # 40 (LAKE), FD&C YELLOW # 6 (LAKE), TRIACETIN.
INDICATIONS
PRENATVITE RX is a multi-vitamin/mineral prescription drug indicated for use in improving the nutritional status of women prior to conception, throughout pregnancy, and in the postnatal period for both lactating and nonlactating mothers.CONTRAINDICATIONS
This product is contraindicated in patients with a known hypersensitivity to any of the ingredients. -
WARNINGS
WARNINGS
Accidental overdose of iron-containing products is a leading cause of fatal poisoning in children under 6. Keep this product out of reach of children. In case of accidental overdose, call a doctor or poison control center immediately.
CAUTION: High levels of folic acid may, especially in older adults, hide signs of Vitamin B-12 deficiency (such as pernicious anemia), a condition that can cause nerve damage.
Folic acid alone is improper therapy in the treatment of pernicious anemia and other megaloblastic anemias where vitamin B is deficient.
PRECAUTIONS
Folic acid in doses above 0.1 mg daily may obscure pernicious anemia in that hematologic remission can occur while neurological manifestations progress.
ADVERSE REACTIONS
Allergic sensitization has been reported following both oral and parenteral administration of folic acid
DOSAGE AND ADMINISTRATIONOne tablet daily or Take exactly as prescribed by your doctor. Take PRENATVITE RX tablet each day at any time of day at about the same time each day. PRENATVITE RX can be taken with or without food.
STORAGE
Store at 20-25°C (68-77°F) [See USP controlled room temperature].
Do not keep medicine that is out of date or that you no longer need.
Keep PRENATVITE RX Tablets and all medicines out of the reach of children.
NOTICE
Contact with moisture can discolor or erode the tablet. -
HEALTH CLAIM
HOW SUPPLIED
Bottles of 90 tablets each - NDC 73317-1050-9
To report a serious adverse event or obtain product information, call 631-524-5758
Call your licensed medical practitioner about side effect.
You may report side effects by calling AUM 1-631-524-5758.
Manufactured and Distributed by:
AUM Pharmaceuticals
Hauppauge, NY 11788.Made in USA
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
PRENATVITE
prenatvite tabletProduct Information Product Type DIETARY SUPPLEMENT Item Code (Source) NHRIC:73317-1050 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength VITAMIN A (UNII: 81G40H8B0T) (VITAMIN A - UNII:81G40H8B0T) VITAMIN A 1200 ug ASCORBIC ACID (UNII: PQ6CK8PD0R) (ASCORBIC ACID - UNII:PQ6CK8PD0R) ASCORBIC ACID 100 mg CHOLECALCIFEROL (UNII: 1C6V77QF41) (CHOLECALCIFEROL - UNII:1C6V77QF41) CHOLECALCIFEROL 10 ug .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) (.ALPHA.-TOCOPHEROL - UNII:H4N855PNZ1) .ALPHA.-TOCOPHEROL ACETATE 4.6 mg THIAMINE (UNII: X66NSO3N35) (THIAMINE ION - UNII:4ABT0J945J) THIAMINE 1.5 mg RIBOFLAVIN (UNII: TLM2976OFR) (RIBOFLAVIN - UNII:TLM2976OFR) RIBOFLAVIN 1.7 mg NIACINAMIDE (UNII: 25X51I8RD4) (NIACINAMIDE - UNII:25X51I8RD4) NIACINAMIDE 18 mg PYRIDOXINE HYDROCHLORIDE (UNII: 68Y4CF58BV) (PYRIDOXINE - UNII:KV2JZ1BI6Z) PYRIDOXINE 2.6 mg FOLIC ACID (UNII: 935E97BOY8) (FOLIC ACID - UNII:935E97BOY8) FOLIC ACID 800 ug CYANOCOBALAMIN (UNII: P6YC3EG204) (CYANOCOBALAMIN - UNII:P6YC3EG204) CYANOCOBALAMIN 4 ug FERROUS FUMARATE (UNII: R5L488RY0Q) (FERROUS CATION - UNII:GW89581OWR) FERROUS CATION 27 mg INOSITOL (UNII: 4L6452S749) (INOSITOL - UNII:4L6452S749) INOSITOL 5 mg CITRUS BIOFLAVONOIDS (UNII: BD70459I50) (HESPERIDIN - UNII:E750O06Y6O) CITRUS BIOFLAVONOIDS 2 mg CALCIUM (UNII: SY7Q814VUP) (CALCIUM - UNII:SY7Q814VUP) CALCIUM 263 mg BORON (UNII: N9E3X5056Q) (BORON - UNII:N9E3X5056Q) BORON 0.2 mg ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC CATION - UNII:13S1S8SF37) ZINC CATION 25 mg Inactive Ingredients Ingredient Name Strength CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) CALCIUM PHOSPHATE, DIBASIC, ANHYDROUS (UNII: L11K75P92J) STEARIC ACID (UNII: 4ELV7Z65AP) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) MAGNESIUM STEARATE (UNII: 70097M6I30) TALC (UNII: 7SEV7J4R1U) POLYETHYLENE GLYCOL 8000 (UNII: Q662QK8M3B) HYPROMELLOSES (UNII: 3NXW29V3WO) TRIACETIN (UNII: XHX3C3X673) FD&C RED NO. 40 (UNII: WZB9127XOA) FD&C YELLOW NO. 6 (UNII: H77VEI93A8) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NHRIC:73317-1050-9 90 in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date DIETARY SUPPLEMENT 02/03/2020 Supplement Facts Serving Size : Serving per Container : Amount Per Serving % Daily Value color scoring 1 shape size (solid drugs) 5 mm Labeler - SLV PHARMACEUTICALS LLC DBA AUM PHARMACEUTICALS (081225162) Establishment Name Address ID/FEI Business Operations SLV PHARMACEUTICALS LLC DBA AUM PHARMACEUTICALS 081225162 repack(73317-1050)