Label: LE-BLEN FGF SERUM- glycerin, sh-polypeptide-11, sodium hyaluronate emulsion
-
Contains inactivated NDC Code(s)
NDC Code(s): 72271-101-01 - Packager: LeBlen Beauty Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated April 25, 2018
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
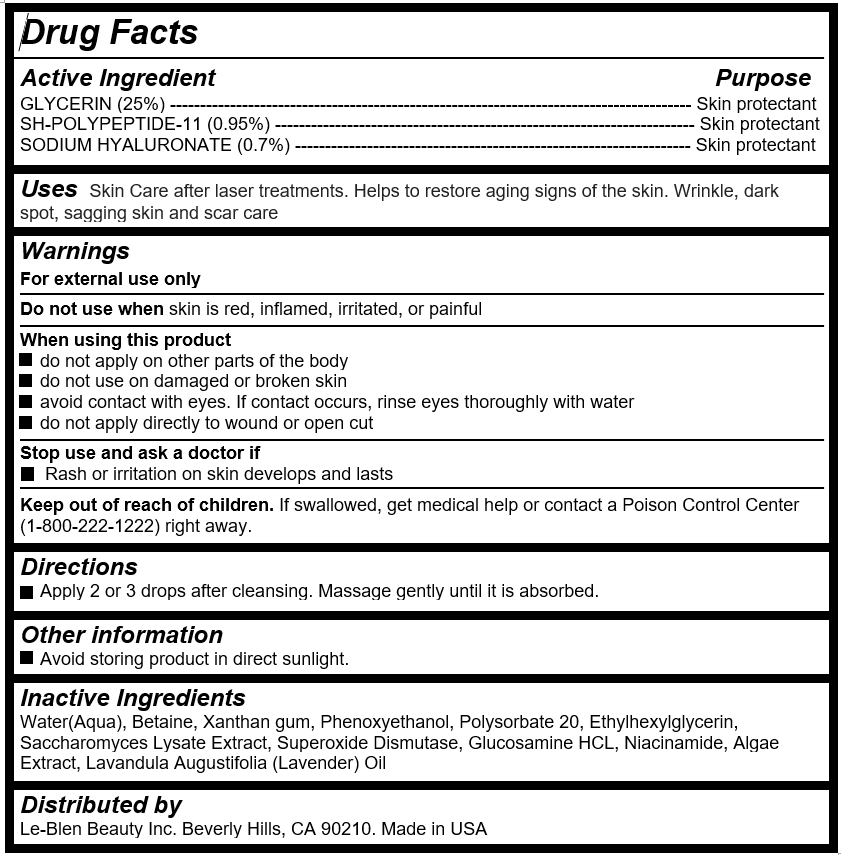
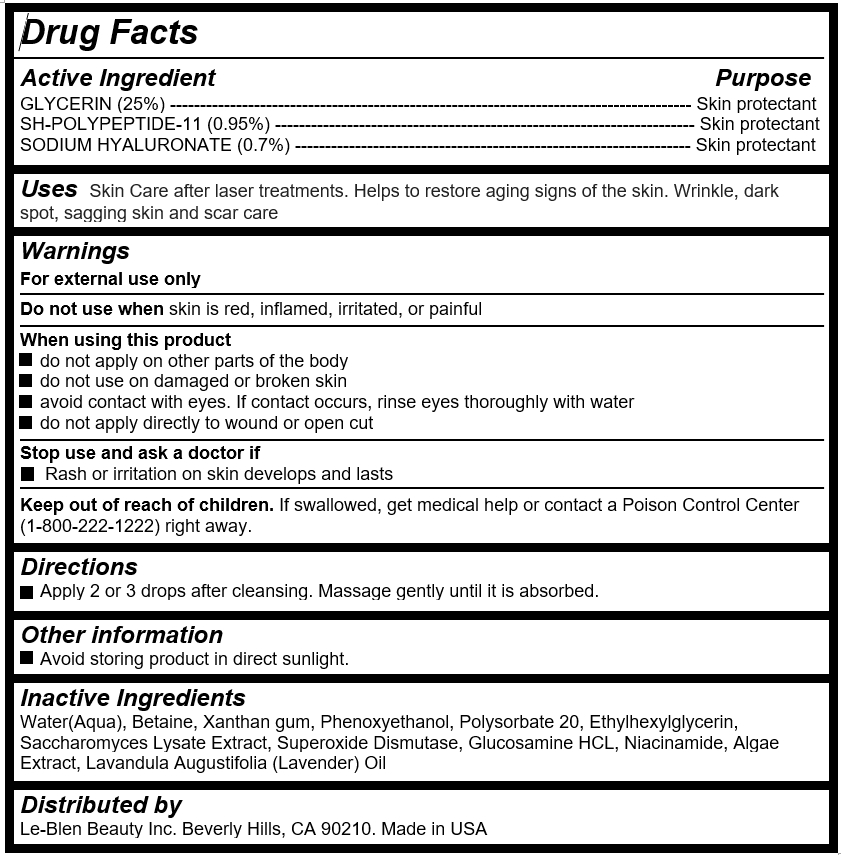
Active Ingredient
GLYCERIN (25%) --------------------------------------------------------------------------------------- Skin protectant
SH-POLYPEPTIDE-11 (0.95%) ---------------------------------------------------------------------- Skin protectant
SODIUM HYALURONATE (0.7%) ------------------------------------------------------------------ Skin protectant
- Purpose
- Uses
-
Warnings
For external use only
Do not use when skin is red, inflamed, irritated, or painful
When using this product
do not apply on other parts of the body
do not use on damaged or broken skin
avoid contact with eyes. If contact occurs, rinse eyes thoroughly with water
do not apply directly to wound or open cut
Stop use and ask a doctor if
Rash or irritation on skin develops and lasts
- Keep out of reach of children
- Directions
- Inactive Ingredients
- Le-Blen FGF Serum
-
INGREDIENTS AND APPEARANCE
LE-BLEN FGF SERUM
glycerin, sh-polypeptide-11, sodium hyaluronate emulsionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:72271-101 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength GLYCERIN (UNII: PDC6A3C0OX) (GLYCERIN - UNII:PDC6A3C0OX) GLYCERIN 14.18 g in 56.7 g FIBROBLAST GROWTH FACTOR-1 (UNII: G53298VN9Y) (FIBROBLAST GROWTH FACTOR-1 - UNII:G53298VN9Y) FIBROBLAST GROWTH FACTOR-1 0.54 g in 56.7 g HYALURONATE SODIUM (UNII: YSE9PPT4TH) (HYALURONIC ACID - UNII:S270N0TRQY) HYALURONATE SODIUM 0.4 g in 56.7 g Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) Product Characteristics Color Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:72271-101-01 56.7 g in 1 BOTTLE, DROPPER; Type 0: Not a Combination Product 04/24/2018 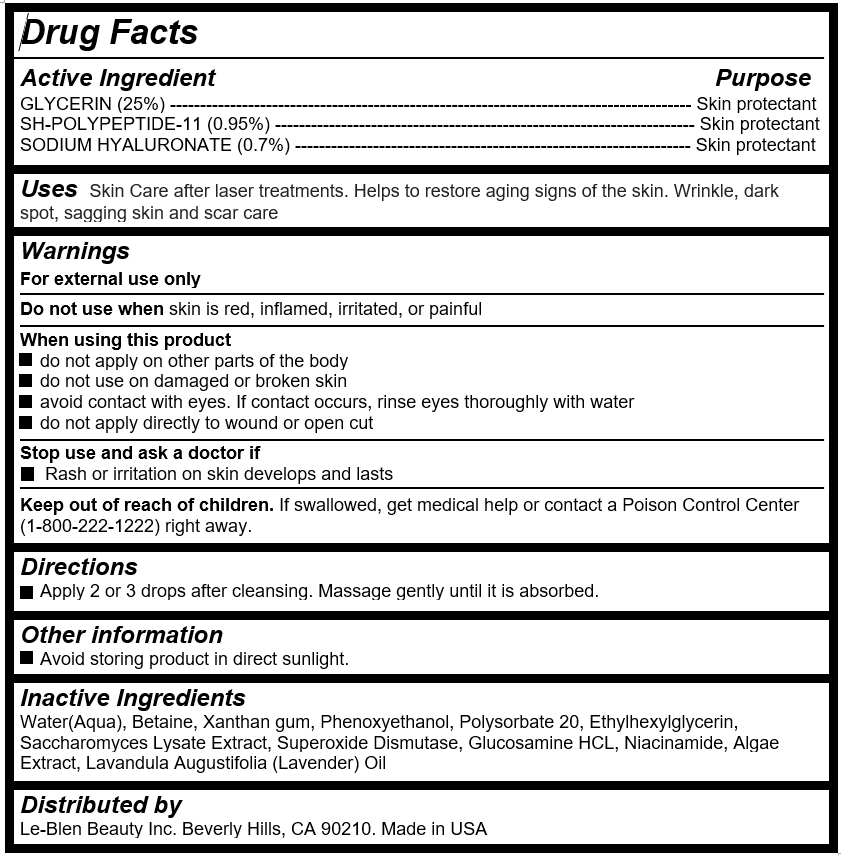
Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 04/24/2018 Labeler - LeBlen Beauty Inc. (091531100) Registrant - LeBlen Beauty Inc. (091531100) Establishment Name Address ID/FEI Business Operations Le-Blen Beauty, Inc. 091531100 label(72271-101) Establishment Name Address ID/FEI Business Operations COSMETIC LAB, INC. 196409143 manufacture(72271-101)