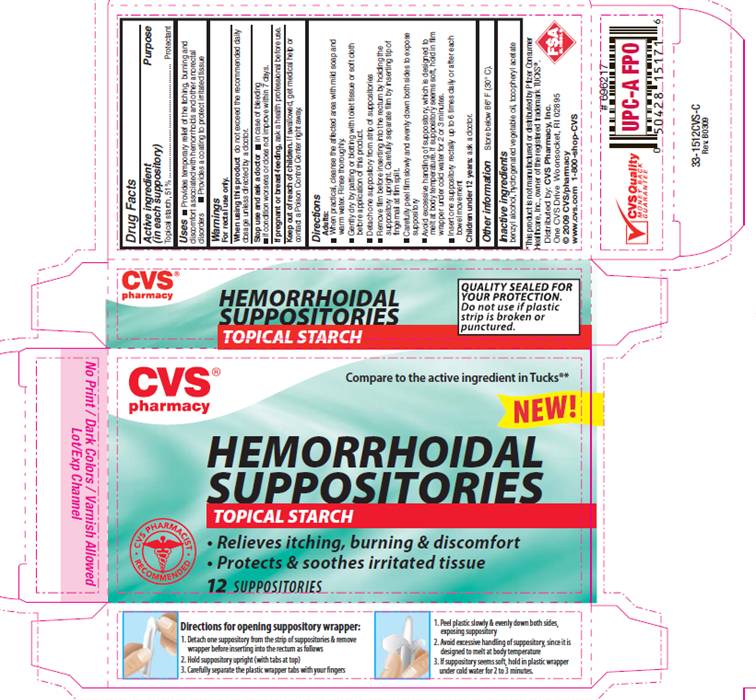
Label: HEMORRHOIDAL STARCH- starch suppository
-
Contains inactivated NDC Code(s)
NDC Code(s): 50730-1512-1 - Packager: H and P Industries, Inc. dba Triad Group
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated December 31, 2009
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- PURPOSE
- USE
- WARNINGS
-
DIRECTIONS
Adults:
- When practical, cleanse the affected area with mild soap and warm water. Rinse thoroughly.
- Gently dry by patting or blotting with toilet tissue or soft cloth before application of this product.
- Detach one suppository from strip of suppositories.
- Remove film before inserting into the rectum by holding the suppository upright. Carefully separate film by inserting tip of fingernail at film split.
- Carefully peel film slowly and evenly down both sides to expose suppository
- Avoid excessive handling of suppository, which is designed to melt at body temperature. If suppository seems soft, hold in film wrapper under cold water for 2 or 3 minutes.
- Insert one suppository rectally up to 6 times daily or after each bowel movement
- SPL UNCLASSIFIED SECTION
- OTHER INFORMATION
- INACTIVE INGREDIENTS
- QUESTIONS
-
PACKAGE INFORMATION - REPRESENTATIVE LABEL
Compare to the active ingredient in TUCKS®*
HEMORRHOIDAL
SUPPOSITORIES
TOPICAL STARCH
- Relieves itching, burning and discomfort
- Protects and soothes irritated tissue
*This product is not manufactured or distributed by Pfizer consumer Healthcare, Inc., owner of the registered trademark TUCKS®.
-
INGREDIENTS AND APPEARANCE
HEMORRHOIDAL STARCH
starch suppositoryProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:50730-1512 Route of Administration RECTAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength STARCH, CORN (UNII: O8232NY3SJ) (STARCH, CORN - UNII:O8232NY3SJ) STARCH, CORN 0.51 g Inactive Ingredients Ingredient Name Strength BENZYL ALCOHOL (UNII: LKG8494WBH) FAT, HARD (UNII: 8334LX7S21) ALPHA-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:50730-1512-1 12 in 1 BOX Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part346 08/01/2006 Labeler - H and P Industries, Inc. dba Triad Group (050259597)