COLD AND FLU SOLUTION- aconitum napellus, anas barbariae, belladonna, echinacea, eupatorium perfoliatum, ferrum phosphoricum, gelsemium sempervirens, liquid
Apotheca Company
Disclaimer: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
----------
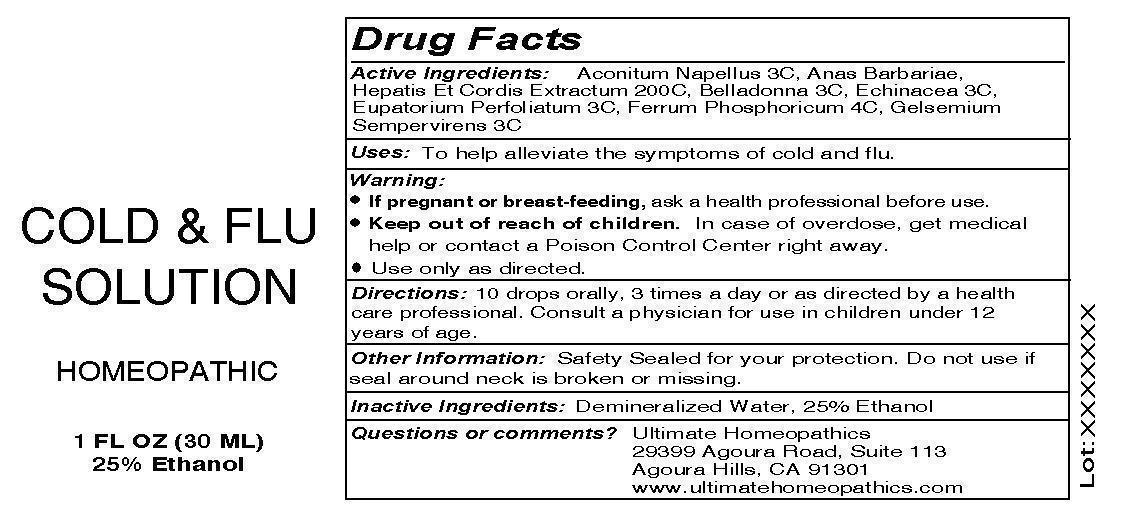
Cold and Flu Solution
Active Ingredients: Aconitum Napellus 3C, Ana Barbariae, Hepatis Et Cordis Extractum 200C, Belladonna 3C, Echinacea 3C, Eupatorium Perfoliatum 3C, Ferrum Phosphoricum 4C, Gelsemium Sempervirens 3C.
WARNING:
* If pregnant or breast-feeding, ask a health professional before use.
* Keep out of reach of children. In case of overdose, get medical help or contact a Poison Control Center right away.
* Use only as directed.
Keep out of reach of children:
Keep out of reach of children. In case of overdose, get medical help or contact a Poison Control Center right away.
Directions: 10 drops orally, 3 times a day or as directed by a health care professional. Consult a physician for use in children under 12 years of age.
Other Information: Safety Sealed for your protection. Do not use if seal around neck is broken or missing.
| COLD AND FLU SOLUTION
aconitum napellus, anas barbariae, belladonna, echinacea, eupatorium perfoliatum, ferrum phosphoricum, gelsemium sempervirens, liquid |
|||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||
| Labeler - Apotheca Company (844330915) |
| Registrant - Apotheca Company (844330915) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Apotheca Company | 844330915 | manufacture(57520-0063) , api manufacture(57520-0063) , label(57520-0063) , pack(57520-0063) | |