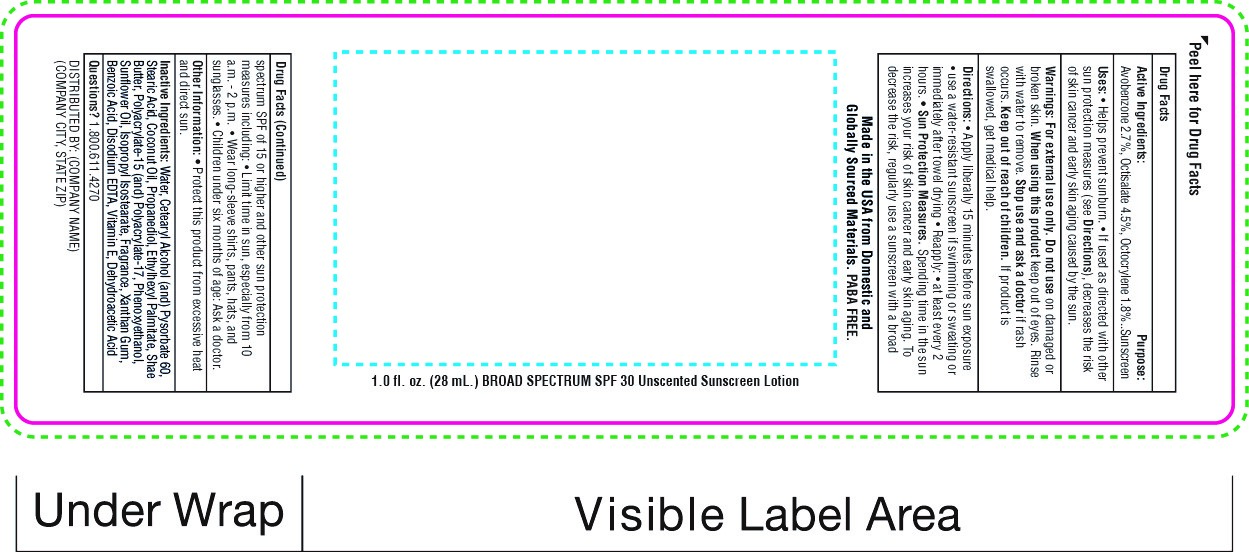
Label: SNUGZ SPF 30 BROAD SPECTRUM SUNSCREEN- spf 30 broad spectrum sunscreen lotion
-
NDC Code(s):
76309-400-01,
76309-400-02,
76309-400-04,
76309-400-08, view more76309-400-19, 76309-400-51, 76309-400-61, 76309-400-62, 76309-400-81, 76309-400-99
- Packager: SnugZ/USA, LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated January 1, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPF 30 Sunscreen Lotion
- SPF 30 Sunscreen Lotion
- SPF 30 Sunscreen Lotion
- SPF 30 Sunscreen Lotion
- SPF 30 Sunscreen Lotion
- SPF 30 Sunscreen Lotion
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
SNUGZ SPF 30 BROAD SPECTRUM SUNSCREEN
spf 30 broad spectrum sunscreen lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:76309-400 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength AVOBENZONE (UNII: G63QQF2NOX) (AVOBENZONE - UNII:G63QQF2NOX) AVOBENZONE 0.000714 mg in 28 mL OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 0.00198 mg in 28 mL OCTOCRYLENE (UNII: 5A68WGF6WM) (OCTOCRYLENE - UNII:5A68WGF6WM) OCTOCRYLENE 0.0018 mg in 28 mL Inactive Ingredients Ingredient Name Strength COCONUT OIL (UNII: Q9L0O73W7L) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:76309-400-01 28 mL in 1 BOTTLE; Type 0: Not a Combination Product 01/01/2018 2 NDC:76309-400-19 56 mL in 1 BOTTLE; Type 0: Not a Combination Product 01/01/2018 3 NDC:76309-400-02 56 mL in 1 BOTTLE; Type 0: Not a Combination Product 01/01/2018 4 NDC:76309-400-04 112 mL in 1 BOTTLE; Type 0: Not a Combination Product 01/01/2018 5 NDC:76309-400-08 226 mL in 1 BOTTLE; Type 0: Not a Combination Product 01/01/2018 6 NDC:76309-400-51 30 mL in 1 TUBE; Type 0: Not a Combination Product 01/01/2018 7 NDC:76309-400-61 39.75 mL in 1 POUCH; Type 0: Not a Combination Product 01/01/2018 8 NDC:76309-400-62 85.17 mL in 1 POUCH; Type 0: Not a Combination Product 01/01/2018 12/31/2020 9 NDC:76309-400-81 28 mL in 1 BOTTLE; Type 0: Not a Combination Product 01/01/2018 10 NDC:76309-400-99 3550 mL in 1 JUG; Type 0: Not a Combination Product 02/10/2021 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 01/01/2018 Labeler - SnugZ/USA, LLC (615959228) Registrant - SnugZ/USA, LLC (615959228) Establishment Name Address ID/FEI Business Operations SnugZ/USA, LLC 615959228 manufacture(76309-400)