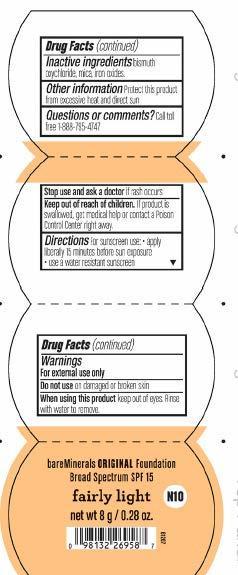
FAIRLY LIGHT FOUNDATION SPF 15 - titanium dioxide powder
Bare Escentuals Beauty Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
BareMineral Original SPF 15 Foundation Fairly Light
| FAIRLY LIGHT FOUNDATION SPF 15
titanium dioxide powder |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Labeler - Bare Escentuals Beauty Inc. (087008363) |
| Registrant - Ei Inc. (105803274) |
Revised: 8/2021
Document Id: c40429ad-4912-4055-aedc-bb207b718745
Set id: 687b3d84-569a-4408-b9b8-3d6a024eee09
Version: 3
Effective Time: 20210820
Bare Escentuals Beauty Inc.