EXUVIANCE COVERBLEND SKIN CARING FOUNDATION SPF 20 BISQUE- titanium dioxide liquid
EXUVIANCE COVERBLEND SKIN CARING FOUNDATION SPF 20 BLUSH BEIGE- titanium dioxide liquid
EXUVIANCE COVERBLEND SKIN CARING FOUNDATION SPF 20 TRUE BEIGE- titanium dioxide liquid
EXUVIANCE COVERBLEND SKIN CARING FOUNDATION SPF 20 NEUTRAL BEIGE- titanium dioxide liquid
EXUVIANCE COVERBLEND SKIN CARING FOUNDATION SPF 20 WARM BEIGE- titanium dioxide liquid
EXUVIANCE COVERBLEND SKIN CARING FOUNDATION SPF 20 HONEY SAND- titanium dioxide liquid
EXUVIANCE COVERBLEND SKIN CARING FOUNDATION SPF 20 DESERT SAND- titanium dioxide liquid
EXUVIANCE COVERBLEND SKIN CARING FOUNDATION SPF 20 CLASSIC BEIGE- titanium dioxide liquid
EXUVIANCE COVERBLEND SKIN CARING FOUNDATION SPF 20 GOLDEN BEIGE- titanium dioxide liquid
EXUVIANCE COVERBLEND SKIN CARING FOUNDATION SPF 20 TOASTED ALMOND- titanium dioxide liquid
NeoStrata Company, Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Exuviance® CoverBlend Skin Caring Foundation
Uses
- Helps prevent sunburn
- If used as directed with other sun protection measures (see Directions), decreases the risk of skin cancer and early skin aging caused by the sun
Directions
- Shake. Blend on evenly with fingertips 15 minutes before sun exposure.
- Use a water resistant sunscreen if swimming or sweating
- Reapply sunscreen at least every 2 hours
-
Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a broad spectrum SPF of 15 or higher and other sun protection measures including:
- Limit time in the sun, especially from 10 a.m. - 2 p.m.
- Wear long-sleeve shirts, pants, hats, and sunglasses
- Children under 6 months: Ask a doctor
Other information
- Store at 15°C - 30°C (59°F - 86°F)
- Protect this product from excessive heat and direct sun
Inactive ingredients
Aqua (Water), Cyclohexasiloxane, Cyclopentasiloxane, Isostearyl Neopentanoate, Talc, Gluconolactone, Isododecane, Butylene Glycol, Isohexadecane, Nylon-12, Polymethyl Methacrylate, Glycerin, Silica, Polyethylene, Sorbitan Sesquioleate, Disteardimonium Hectorite, PEG-30 Dipolyhydroxystearate, Sodium Chloride, Lactobionic Acid, Tocopheryl Linoleate/Oleate, Lecithin, Polyglyceryl-4 Isostearate, Hexyl Laurate, Stearic Acid, Aluminum Hydroxide, Propylene Carbonate, PEG/PPG-18/18 Dimethicone, Perfluorooctyl Triethoxysilane, Ammonium C6-16 Perfluoroalkylethyl Phosphate, Cetyl PEG/PPG-10/1 Dimethicone, Ammonium Hydroxide, Magnesium Stearate, Polymethylsilsesquioxane, Phenoxyethanol, Chlorphenesin. MAY CONTAIN: CI 77492 (Iron Oxide Yellow), CI 77491 (Iron Oxide Red), CI 77499 (Iron Oxide Black)
PRINCIPAL DISPLAY PANEL - 30 mL Bottle Carton - Bisque
DERMATOLOGIST DEVELOPED
Exuviance®
CB
CoverBlend
Skin Caring Foundation
with Sunscreen Broad Spectrum
SPF 20
Light to medium, all-day coverage
+
Powerful antiaging benefits
30 mL / 1.0 fl oz

PRINCIPAL DISPLAY PANEL - 30 mL Bottle Carton - Blush Beige
DERMATOLOGIST DEVELOPED
Exuviance®
CB
CoverBlend
Skin Caring Foundation
with Sunscreen Broad Spectrum
SPF 20
Light to medium, all-day coverage
+
Powerful antiaging benefits
30 mL / 1.0 fl oz

PRINCIPAL DISPLAY PANEL - 30 mL Bottle Carton - True Beige
DERMATOLOGIST DEVELOPED
Exuviance®
CB
CoverBlend
Skin Caring Foundation
with Sunscreen Broad Spectrum
SPF 20
Light to medium, all-day coverage
+
Powerful antiaging benefits
30 mL / 1.0 fl oz

PRINCIPAL DISPLAY PANEL - 30 mL Bottle Carton - Neutral Beige
DERMATOLOGIST DEVELOPED
Exuviance®
CB
CoverBlend
Skin Caring Foundation
with Sunscreen Broad Spectrum
SPF 20
Light to medium, all-day coverage
+
Powerful antiaging benefits
30 mL / 1.0 fl oz

PRINCIPAL DISPLAY PANEL - 30 mL Bottle Carton - Warm Beige
DERMATOLOGIST DEVELOPED
Exuviance®
CB
CoverBlend
Skin Caring Foundation
with Sunscreen Broad Spectrum
SPF 20
Light to medium, all-day coverage
+
Powerful antiaging benefits
30 mL / 1.0 fl oz

PRINCIPAL DISPLAY PANEL - 30 mL Bottle Carton - Honey Sand
DERMATOLOGIST DEVELOPED
Exuviance®
CB
CoverBlend
Skin Caring Foundation
with Sunscreen Broad Spectrum
SPF 20
Light to medium, all-day coverage
+
Powerful antiaging benefits
30 mL / 1.0 fl oz

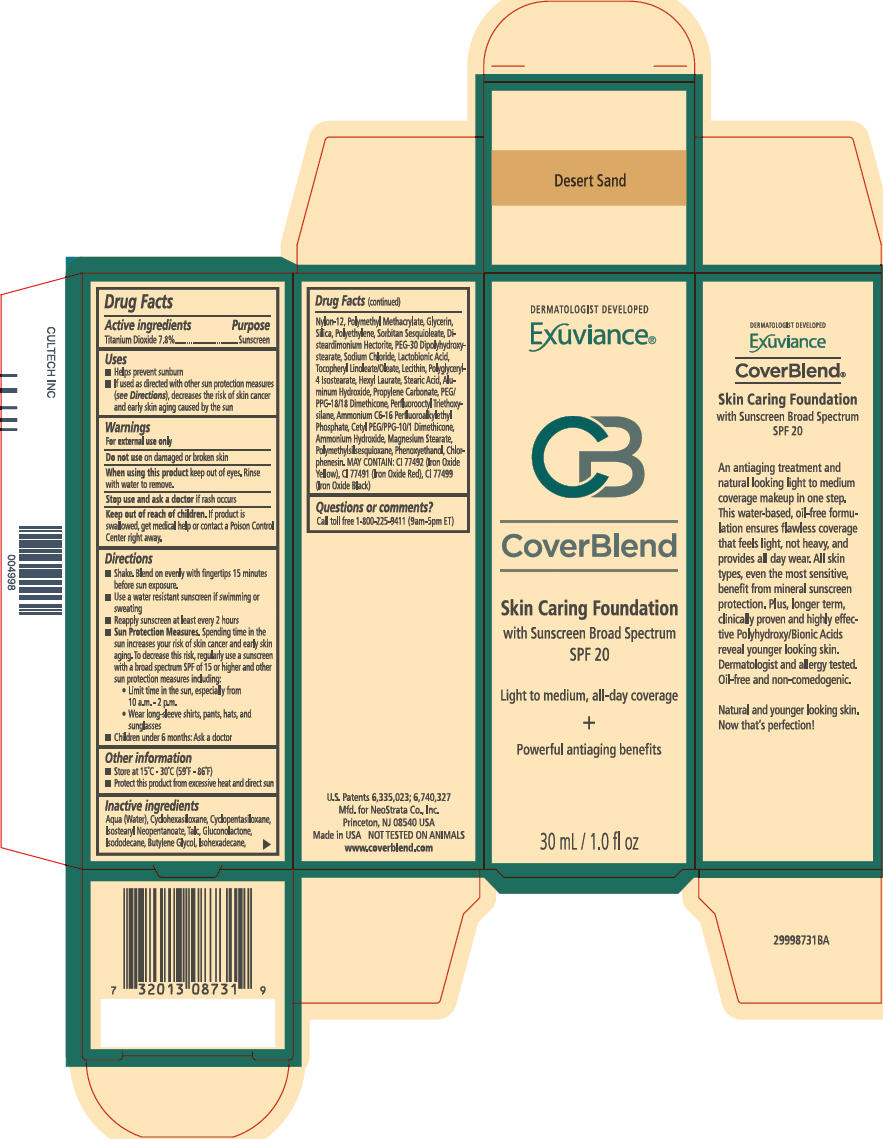
PRINCIPAL DISPLAY PANEL - 30 mL Bottle Carton - Desert Sand
DERMATOLOGIST DEVELOPED
Exuviance®
CB
CoverBlend
Skin Caring Foundation
with Sunscreen Broad Spectrum
SPF 20
Light to medium, all-day coverage
+
Powerful antiaging benefits
30 mL / 1.0 fl oz
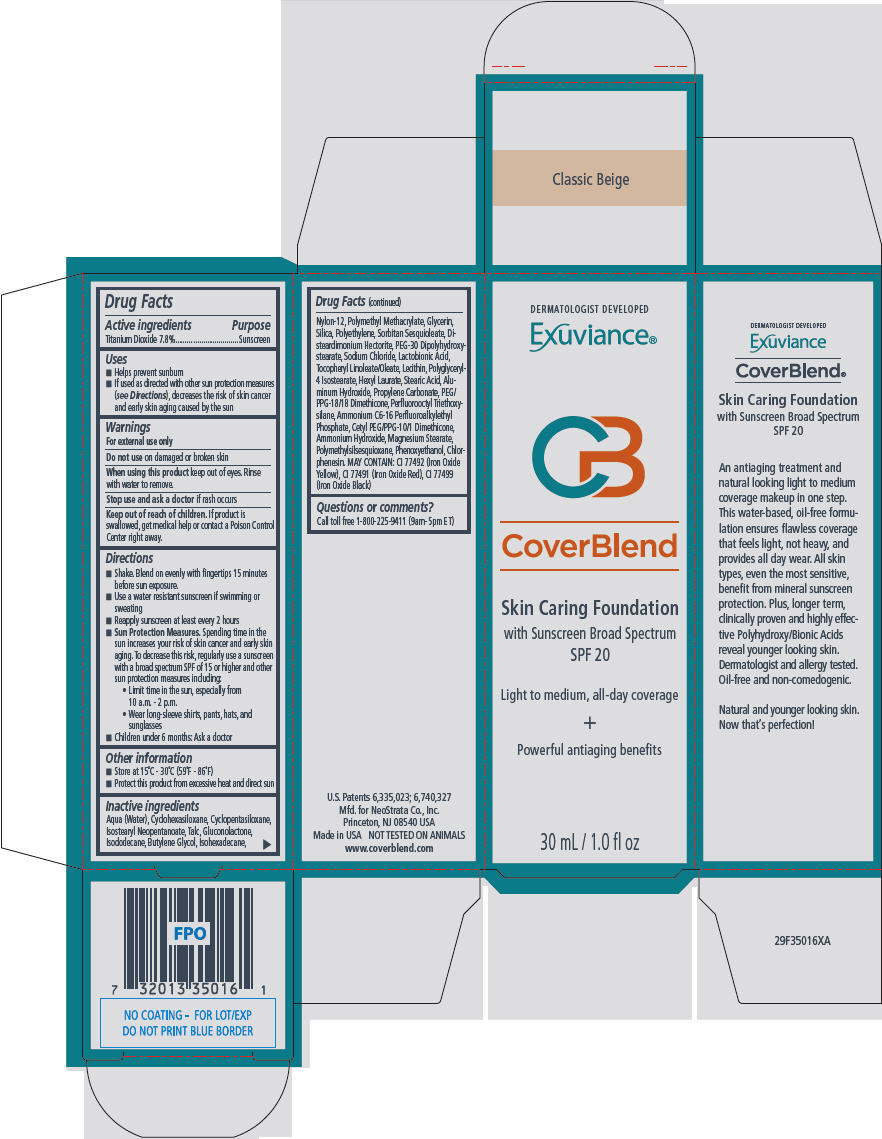
PRINCIPAL DISPLAY PANEL - 30 mL Bottle Carton - Classic Beige
DERMATOLOGIST DEVELOPED
Exuviance®
CB
CoverBlend
Skin Caring Foundation
with Sunscreen Broad Spectrum
SPF 20
Light to medium, all-day coverage
+
Powerful antiaging benefits
30 mL / 1.0 fl oz
| EXUVIANCE COVERBLEND SKIN CARING FOUNDATION
SPF 20 BISQUE
titanium dioxide liquid |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| EXUVIANCE COVERBLEND SKIN CARING FOUNDATION
SPF 20 BLUSH BEIGE
titanium dioxide liquid |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| EXUVIANCE COVERBLEND SKIN CARING FOUNDATION
SPF 20 TRUE BEIGE
titanium dioxide liquid |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| EXUVIANCE COVERBLEND SKIN CARING FOUNDATION
SPF 20 NEUTRAL BEIGE
titanium dioxide liquid |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| EXUVIANCE COVERBLEND SKIN CARING FOUNDATION
SPF 20 WARM BEIGE
titanium dioxide liquid |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| EXUVIANCE COVERBLEND SKIN CARING FOUNDATION
SPF 20 HONEY SAND
titanium dioxide liquid |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| EXUVIANCE COVERBLEND SKIN CARING FOUNDATION
SPF 20 DESERT SAND
titanium dioxide liquid |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| EXUVIANCE COVERBLEND SKIN CARING FOUNDATION
SPF 20 CLASSIC BEIGE
titanium dioxide liquid |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| EXUVIANCE COVERBLEND SKIN CARING FOUNDATION
SPF 20 GOLDEN BEIGE
titanium dioxide liquid |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| EXUVIANCE COVERBLEND SKIN CARING FOUNDATION
SPF 20 TOASTED ALMOND
titanium dioxide liquid |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - NeoStrata Company, Inc. (605754829) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Kolmar Laboratories | 001535103 | MANUFACTURE(58414-0001, 58414-0002, 58414-0003, 58414-0004, 58414-0005, 58414-0006, 58414-0007, 58414-0008, 58414-0009, 58414-0010) | |

